 | |
| Names | |
|---|---|
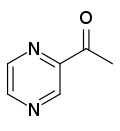
| Preferred IUPAC name
1-(Pyrazin-2-yl)ethan-1-one | |
| Other names
2-Acetylpyrazine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.040.670 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H6N2O | |
| Molar mass | 122.127 g·mol−1 |
| Appearance | Yellow-brown powder |
| Melting point | 75–78 °C (167–172 °F; 348–351 K)[1] |
| Boiling point | 78–79 °C (172–174 °F; 351–352 K) (8 mmHg)[2] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Irritant |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Acetylpyrazine is an organic compound with the chemical formula C
6H
6N
2O. It is a yellow-brown powder at room temperature.[2] Chemically, acetylpyrazine is a pyrazine and a ketone.[1]
Natural occurrence
Acetylpyrazine is found in foods such as seeds, nuts and meats.
Uses
It is used in frozen dairy products such as ice cream.
It is considered generally recognized as safe by the U.S. Food and Drug Administration.[3]
"Essence formula for increasing cigarette fragrance and improving smoke quality".[4]
It is also known to be part of the formulation of e-cigarettes (vapes):[5]
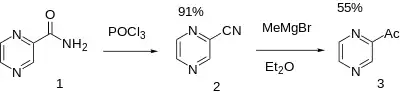
Synthesis
Note that modern synthesis is for 2-cyanopyrazine from 2-methylpyrazine [109-08-0].[6][7]
Pyrazinamide [98-96-4] (1) 2-Cyanopyrazine [19847-12-2] (2)
More modern syntheses have also been reported in recent years:[10][11][12]
References
- 1 2 Acetylpyrazine on Sigma Aldrich
- 1 2 Acetylpyrazine on Chemical Book
- ↑ Martin, Terry (8 Feb 2004). "Acetylpyrazine". About.com Smoking Cessation. Archived from the original on 6 January 2016. Retrieved 16 June 2014.
- ↑ Mao Deshou, et al. CN115216363 (2022 to China Tobacco Yunnan Industrial Co Ltd).
- ↑ Public Health Consequences of E-Cigarettes. NAP.edu.
- ↑ Rao, K. Narasimha; Gopinath, Rajesh; Prasad, P. S. Sai (2001). "Highly selective molybdenum phosphate catalyst for the ammoxidation of 2-methylpyrazine to 2-cyanopyrazine". Green Chemistry. 3 (1): 20–22. doi:10.1039/b005643j.
- ↑ Hong, Chun; Li, Yong (2006). "2-Cyanopyrazine Prepared from 2-Methylpyrazine by Catalytic Ammoxidation on MoVPO Catalyst". Chinese Journal of Chemical Engineering. 14 (5): 670–675. doi:10.1016/S1004-9541(06)60133-X.
- ↑ Donald L Roberts, U.S. Patent 3,402,051 (1968 to RJ Reynolds Tobacco Co).
- ↑ Jr Victor K Smith & Kushner Samuel, U.S. Patent 2,677,686 (1954 to Wyeth Holdings LLC).
- ↑ Mao, Lin; Niu, Dong-Fang; Hu, Shuo-Zhen; Zhang, Xin-Sheng (2022-05-28). "Electrochemical Synthesis of Acetylpyrazine". Journal of Electrochemistry. 28 (5): 2107061. doi:10.13208/j.electrochem.210706.
- ↑ Wang et al.: Progress in the Synthesis and Application of Acetylpyrazine, 2011.
- ↑ 任伟, et al. CN106588785 (2017 to Yoshida Spice Ltd By Share Ltd Shandong).