| ACBP | |||||||||
|---|---|---|---|---|---|---|---|---|---|
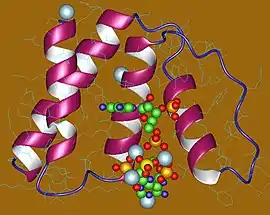 Acyl-CoA-binding protein monomer, Human | |||||||||
| Identifiers | |||||||||
| Symbol | ACBP | ||||||||
| Pfam | PF00887 | ||||||||
| InterPro | IPR000582 | ||||||||
| PROSITE | PDOC00686 | ||||||||
| SCOP2 | 1aca / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 295 | ||||||||
| OPM protein | 2wh5 | ||||||||
| CDD | cd00435 | ||||||||
| Membranome | 497 | ||||||||
| |||||||||
In molecular biology, the Acyl-CoA-binding protein (ACBP) is a small (10 Kd) protein that binds medium- and long-chain acyl-CoA esters with very high affinity and may function as an intracellular carrier of acyl-CoA esters.[1] ACBP is also known as diazepam binding inhibitor (DBI) or endozepine (EP) because of its ability to displace diazepam from the benzodiazepine (BZD) recognition site located on the GABA type A receptor. It is therefore possible that this protein also acts as a neuropeptide to modulate the action of the GABA receptor.[2]
ACBP is a highly conserved protein of about 90 amino acids that is found in all four eukaryotic kingdoms, Animalia, Plantae, Fungi and Protista, and in some eubacterial species.[3]
Although ACBP occurs as a completely independent protein, intact ACB domains have been identified in a number of large, multifunctional proteins in a variety of eukaryotic species. These include large membrane-associated proteins with N-terminal ACB domains, multifunctional enzymes with both ACB and peroxisomal enoyl-CoA Delta(3), Delta(2)-enoyl-CoA isomerase domains, and proteins with both an ACB domain and ankyrin repeats.[3]
The ACB domain consists of four alpha-helices arranged in a bowl shape with a highly exposed acyl-CoA-binding site. The ligand is bound through specific interactions with residues on the protein, most notably several conserved positive charges that interact with the phosphate group on the adenosine-3'phosphate moiety, and the acyl chain is sandwiched between the hydrophobic surfaces of CoA and the protein.[4]
Other proteins containing an ACB domain include:
- Endozepine-like peptide (ELP) (gene DBIL5) from mouse.[5] ELP is a testis-specific ACBP homologue that may be involved in the energy metabolism of the mature sperm.
- MA-DBI, a transmembrane protein of unknown function which has been found in mammals. MA-DBI contains a N-terminal ACB domain.
- DRS-1,[6] a human protein of unknown function that contains a N-terminal ACB domain and a C-terminal enoyl-CoA isomerase/hydratase domain.
References
- ↑ Rose TM, Schultz ER, Todaro GJ (December 1992). "Molecular cloning of the gene for the yeast homolog (ACB) of diazepam binding inhibitor/endozepine/acyl-CoA-binding protein". Proc. Natl. Acad. Sci. U.S.A. 89 (23): 11287–91. doi:10.1073/pnas.89.23.11287. PMC 50535. PMID 1454809.
- ↑ Costa E, Guidotti A (1991). "Diazepam binding inhibitor (DBI): a peptide with multiple biological actions". Life Sci. 49 (5): 325–44. doi:10.1016/0024-3205(91)90440-M. PMID 1649940.
- 1 2 Burton M, Rose TM, Faergeman NJ, Knudsen J (December 2005). "Evolution of the acyl-CoA binding protein (ACBP)". Biochem. J. 392 (Pt 2): 299–307. doi:10.1042/BJ20050664. PMC 1316265. PMID 16018771.
- ↑ van Aalten DM, Milne KG, Zou JY, Kleywegt GJ, Bergfors T, Ferguson MA, Knudsen J, Jones TA (May 2001). "Binding site differences revealed by crystal structures of Plasmodium falciparum and bovine acyl-CoA binding protein". J. Mol. Biol. 309 (1): 181–92. doi:10.1006/jmbi.2001.4749. PMID 11491287.
- ↑ Pusch W, Balvers M, Hunt N, Ivell R (August 1996). "A novel endozepine-like peptide (ELP) is exclusively expressed in male germ cells". Mol. Cell. Endocrinol. 122 (1): 69–80. doi:10.1016/0303-7207(96)03874-9. PMID 8898349. S2CID 36504570.
- ↑ Suk K, Kim YH, Hwang DY, Ihm SH, Yoo HJ, Lee MS (May 1999). "Molecular cloning and expression of a novel human cDNA related to the diazepam binding inhibitor". Biochim. Biophys. Acta. 1454 (1): 126–31. doi:10.1016/s0925-4439(99)00033-2. PMID 10354522.