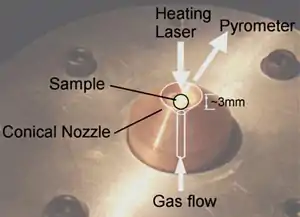

Aerodynamic levitation is the use of gas pressure to levitate materials so that they are no longer in physical contact with any container. In scientific experiments this removes contamination and nucleation issues associated with physical contact with a container.
Overview
The term aerodynamic levitation could be applied to many objects that use gas pressure to counter the force of gravity, and allow stable levitation. Helicopters and air hockey pucks are two good examples of objects that are aerodynamically levitated. However, more recently this term has also been associated with a scientific technique which uses a cone-shaped nozzle allowing stable levitation of 1-3mm diameter spherical samples without the need for active control mechanisms.[1]
Aerodynamic levitation as a scientific tool
These systems allow spherical samples to be levitated by passing gas up through a diverging conical nozzle. Combining this with >200W continuous CO2 laser heating allows sample temperatures in excess of 3000 degrees Celsius to be achieved.
When heating materials to these extremely high temperatures levitation in general provides two key advantages over traditional furnaces. First, contamination that would otherwise occur from a solid container is eliminated. Second, the sample can be undercooled, i.e. cooled below its normal freezing temperature without actually freezing.
Undercooling of liquid samples
Undercooling, or supercooling, is the cooling of a liquid below its equilibrium freezing temperature while it remains a liquid. This can occur wherever crystal nucleation is suppressed. In levitated samples, heterogeneous nucleation is suppressed due to lack of contact with a solid surface. Levitation techniques typically allow samples to be cooled several hundred degrees Celsius below their equilibrium freezing temperatures.
Glass produced by aerodynamic levitation
Since crystal nucleation is suppressed by levitation, and since it is not limited by sample conductivity (unlike electromagnetic levitation), aerodynamic levitation can be used to make glassy materials, from high temperature melts that cannot be made by standard methods. Several silica-free, aluminium oxide based glasses have been made.[2][3][4]
Physical property measurements
In the last few years a range of in situ measurement techniques have also been developed. The following measurements can be made with varying precision:
electrical conductivity, viscosity,[5] density, surface tension,[6] specific heat capacity,
In situ aerodynamic levitation has also been combined with:
X-ray synchrotron radiation, neutron scattering, NMR spectroscopy
See also
Further reading
- Price, D.L. (2010). High-Temperature Levitated Materials. Cambridge University Press. ISBN 978-0521880527.
References
- ↑ Paul C. Nordine; J. K. Richard Weber & Johan G. Abadie (2000), "Properties of high-temperature melts using levitation", Pure and Applied Chemistry, 72 (11): 2127–2136, doi:10.1351/pac200072112127
- ↑ J. K. Richard Weber; Jean A. Tangeman; Thomas S. Key; Kirsten J. Hiera; Paul-Francois Paradis; Takehiko Ishikawa; et al. (2002), "Novel Synthesis of Calcium Oxide–Aluminum Oxide Glasses", Japanese Journal of Applied Physics, 41 (5A): 3029–3030, Bibcode:2002JaJAP..41.3029W, doi:10.1143/JJAP.41.3029
- ↑ J. K. Richard Weber; Johan G. Abadie; April D. Hixson; Paul C. Nordine; Gregory A. Jerman (2004), "Glass Formation and Polyamorphism in Rare-Earth Oxide–Aluminum Oxide Compositions", Journal of the American Ceramic Society, 83 (8): 1868–1872, doi:10.1111/j.1151-2916.2000.tb01483.x
- ↑ L. B. Skinner; A. C. Barnes & W. Crichton (2006), "Novel behaviour and structure of new glasses of the type Ba–Al–O and Ba–Al–Ti–O produced by aerodynamic levitation and laser heating", Journal of Physics: Condensed Matter, 18 (32): L407–L414, Bibcode:2006JPCM...18L.407S, doi:10.1088/0953-8984/18/32/L01, PMID 21690853
- ↑ Kondo, Toshiki; Muta, Hiroaki; Kurosaki, Ken; Kargl, Florian; Yamaji, Akifumi; Furuya, Masahiro; Ohishi, Yuji (July 2019). "Density and viscosity of liquid ZrO2 measured by aerodynamic levitation technique". Heliyon. 5 (7): e02049. doi:10.1016/j.heliyon.2019.e02049. PMC 6658727.
- ↑ Sun, Yifan; Muta, Hiroaki; Ohishi, Yuji (June 2021). "Novel Method for Surface Tension Measurement: the Drop-Bounce Method". Microgravity Science and Technology. 33 (3): 32. doi:10.1007/s12217-021-09883-7.