 | |
| Names | |
|---|---|
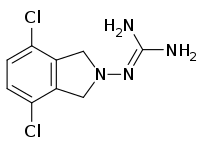
| IUPAC name
2-(4,7-Dichloro-1,3-dihydroisoindol-2-yl)guanidine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H10Cl2N4 | |
| Molar mass | 245.109 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Aganodine is a guanidine that activates presynaptic imidazoline receptors.[1] Through its agonism at imidazoline receptors, aganodine inhibits the presynaptic release of norepinephrine.[2]
References
- ↑ Molderings, GJ; Likungu, J; Jakschik, J; Göthert, M (1997). "Presynaptic imidazoline receptors and non-adrenoceptor 3H-idazoxan binding sites in human cardiovascular tissues". British Journal of Pharmacology. 122 (1): 43–50. doi:10.1038/sj.bjp.0701343. PMC 1564902. PMID 9298527.
- ↑ Göthert, M.; Brüss, M.; Bönisch, H.; Molderings, G.J. (1999). "Presynaptic Imidazoline Receptors". Annals of the New York Academy of Sciences. 881: 171–184. doi:10.1111/j.1749-6632.1999.tb09356.x. PMID 10415912. S2CID 12242403.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.