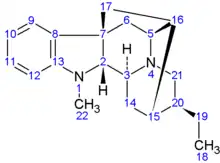
 The conventional representation of the ajmalan skeleton, with numbering | |
| Names | |
|---|---|
| IUPAC name
(1S,9R,10S,12S,13S,16S,17S)-13-Ethyl-8-methyl-8,15-diazahexacyclo[14.2.1.01,9.02,7.010,15.012,17]nonadeca-2,4,6-triene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| |
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Ajmalan is a parent hydride used in the IUPAC nomenclature of natural products and also in CAS nomenclature.[1] It is a 20-carbon alkaloid with six rings and seven chiral centres.
The name is derived from ajmaline, an antiarrhythmic alkaloid isolated from the roots of Rauvolfia serpentina[2] which is formally a dihydroxy-derivative of ajmalan. The –an ending indicates that ajmalan is partially saturated. Ajmaline itself is named after Hakim Ajmal Khan, a distinguished practitioner of the Unani school of traditional medicine in South Asia.[3]
The absolute configuration of the seven chiral carbon atoms in ajmalan is defined by convention, as is the numbering system.[1] The stereochemistry is the same as that in naturally occurring ajmaline, and corresponds to (2R,3S,5S,7S,15S,16R,20S) using conventional numbering.
Ajmalan can be systematically named as
- (1S,4S,5S,7S,8R,16S,17R)-4-ethyl-9-methyl-2,9-diazahexacyclo[14.2.1.02,7.05,18.08,16.010,15]nonadeca-10,12,14-triene
or as
- (2S,3S,5S,6aS,11aR,11bS,12R)-4H,11H-3-ethyl-11-methyl-1,2,3,5,6,6a,11a,11b-octahydro-2,5,6a-(epiethane[1,1,2]triyl)indolo[2,3-c]quinolizine.
Note that the numbering of the atoms in the systematic names is different from the conventional numbering of ajmalan.[4]
The ajmalan skeleton is similar to those of certain other alkaloids, and ajmalan could also be given the following semisystematic names:
- (2β,5β,16R,20β)-1-methyl-1,2,19,20-tetrahydro-5,16-cyclo-16a-homo-17-norakuammilan;
- (2β,5β,7β,16R,20β)-1-methyl-2,7-dihydro-5,16:7,17-dicyclocorynan;
- (2β,7β,16R,20β)-1-methyl-2,7,19,20-tetrahydro-7,17-cyclosarpagan;
- (2β,3α,7β,20β)-1-methyl-2,7,19,20-tetrahydro-3,4:7,17-dicyclo-22-norvobasan;
- (2β,5β,7β,16R,20β)-1-methyl-2,7-dihydro-5,16:7,17-dicyclo-17-secoyohimban.
However, the relative complexity even of these names justifies the use of ajmalan as a defined parent hydride in alkaloid nomenclature.
References
- 1 2 "Revised Section F: Natural Products and Related Compounds (IUPAC Recommendations 1999)", Pure Appl. Chem., 71 (4): 587–643, doi:10.1351/pac199971040587.
- ↑ Siddiqui, S.; Siddiqui, R. H. (1931). J. Indian Chem. Soc. 8: 667–80.
- ↑ Ahmed Nasim Sandilvi (2003). "Salimuzzaman Siddiqui: pioneer of scientific research in Pakistan. Archived 2007-09-27 at the Wayback Machine" Daily Dawn, 2003-04-12. Retrieved on 2007-07-19.
- ↑ The numbering is also different between the von Baeyer name and the fusion name, given the different conventions which apply to the two methods of nomenclature.