 | |
 | |
 | |
| Names | |
|---|---|
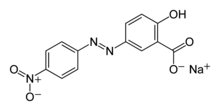
| IUPAC name
Sodium 2-hydroxy-5-[(E)-(4-nitrophenyl)diazenyl]benzoate | |
| Other names
5-[(p-Nitrophenyl)azo]salicylic acid sodium salt Chrome orange Mordant orange 1 C.I. 14030 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.017.109 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
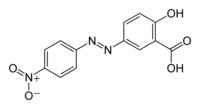
| C13H8N3NaO5 (Na salt) C13H9N3O5 (acid) | |
| Molar mass | 309.21 g mol−1 (Na salt) 287.23 g mol−1 (acid) |
| HazardsSigma-Aldrich Co., ALIZARINE YELLOW R. Retrieved on 09 April 2023. | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H319 | |
| P264, P270, P280, P301+P312, P305+P351+P338, P330, P337+P313, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
| Alizarine Yellow R (pH indicator) | ||
| below pH 10.1 | above pH 12.0 | |
| 10.1 | ⇌ | 12.0 |
Alizarine Yellow R is a yellow colored azo dye made by the diazo coupling reaction. It is usually commercially available as a sodium salt. In its pure form, it is a rust-colored solid.[2] It is mainly used as a pH indicator.
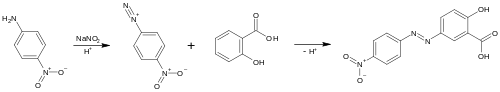
Preparation
Alizarine Yellow R is produced by azo coupling of salicylic acid and diazonium derivative of 4-Nitroaniline
References
- ↑ Lide, David R. (25 June 2007). CRC Handbook of Chemistry and Physics, 88th Edition. CRC Press. pp. 3–10. ISBN 9780849304880. OCLC 1024315229.
- ↑ "Safety Datasheet (MSDS) for alizarin yellow R". Department of Chemistry, University of Oxford. 2005. Archived from the original on 19 March 2011. Retrieved 11 October 2008.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.