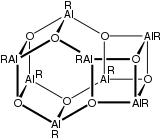
Octa-aluminoxane (R = tert-Bu).
Aluminoxanes are organoaluminium compounds with the formula [RAlO]m[R2AlO0.5]n[R2AlOH]o, where R = organic substituent. The following structural rules apply: Al is tetrahedral and O is three-coordinate.[1][2]
Methylaluminoxane is widely used in the polymerization of alkenes. These compounds are typically obtained by the partial hydrolysis of trialkylaluminium compounds. Aluminoxanes serve as activators for catalytic olefin polymerisation, such as the Ziegler–Natta catalyst. They also serve a function as scavenger for impurities (e.g. water) in reactions that are sensitive to these impurities. Aluminoxane, appearing as white solids, are encountered as solutions.
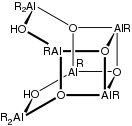
Aluminoxane with OH groups (R = tert-Bu).
References
- ↑ Harlan, C. Jeff; Mason, Mark R.; Barron, Andrew R. (1994). "Tert-Butylaluminum Hydroxides and Oxides: Structural Relationship between Alkylalumoxanes and Alumina Gels". Organometallics. 13 (8): 2957–2969. doi:10.1021/om00020a011.
- ↑ Mason, Mark R.; Smith, Janna M.; Bott, Simon G.; Barron, Andrew R. (1993). "Hydrolysis of tri-tert-Butylaluminum: The First Structural Characterization of Alkylalumoxanes [(R2Al)2O]n and (RAlO)n". Journal of the American Chemical Society. 115 (12): 4971–4984. doi:10.1021/ja00065a005.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.