| |||
| Names | |||
|---|---|---|---|
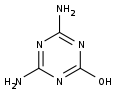
| Preferred IUPAC name
4,6-Diamino-1,3,5-triazin-2-ol | |||
| Other names
2,4-Diamino-6-hydroxy-1,3,5-triazine 4,6-Diamino-2-hydroxy-1,3,5-triazine 4,6-diamino-1,3,5-triazin-2(1H)-one | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.010.415 | ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C3H5N5O | |||
| Molar mass | 127.107 g·mol−1 | ||
| Appearance | White powder | ||
| Melting point | N/A (decomposes before melting) | ||
| Trace | |||
| Solubility | Soluble in aqueous alkalies and mineral acids, but not acetic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Ammeline (4,6-diamino-2-hydroxy-1,3,5-triazine) is a triazine derivative. It is the hydrolysis product of melamine.[1]
Synthesis
Ammeline can be synthesized by the pyrolysis of urea or the condensation reaction among 2 moles of dicyandiamide and 1 mole of biuret.
- 2 C2H4N4 + C2H5N3O2 → 2C3H5N5O + NH3
Chemical properties
Ammeline is weakly acidic with pKa ~9. It can form nitrate, sulfate, chromate, and oxalate salts. Ammeline reacts with boiling dilute hydrochloric acid to form melem and ammonia.
Ammeline is the first step in melamine hydrolysis. Further hydrolysis (e.g. boiling ammeline with dilute alkali) yields ammelide.
References
- ↑ B. Bann and S.A. Miller (1958). "Melamines and derivatives of melamine". Chemical Reviews. 58: 131–172. doi:10.1021/cr50019a004.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.