 | |
| Names | |
|---|---|
| IUPAC name
Diuranyl heptoxide | |
| Other names
Amorphous UO3 | |
| Properties | |
| Am-U2O7 | |
| Molar mass | 588 g/mol |
| Appearance | Orange-brown powder |
| Density | 6.8 g/cm3 |
| Partially soluble | |
| Related compounds | |
| Uranyl peroxide Triuranium octoxide | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Amorphous uranium(VI) oxide (am-U2O7) is an orange diuranyl compound, most commonly obtained from the thermal decomposition of uranyl peroxide tetrahydrate at temperatures between 150 and 500 °C (300 and 930 °F). It exists at room temperature as a powder. Am-U2O7 does not comprise a regular, long-range atomic structure, as demonstrated by its characteristic diffuse scattering pattern obtained by X-ray diffraction. As a result, the molecular structure of this material is little understood, although experimental and computational attempts to elucidate a local atomic environment have yielded some success.[2][3]
Production
Am-U2O7 is produced by the thermal decomposition of uranyl peroxide tetrahydrate at temperatures between 150 and 500 °C (300 and 930 °F), in either an air or nitrogen atmosphere. The resultant powder is tan orange in colour. Further heating results in the formation of alpha uranium trioxide (α-UO3).
Structure and reactivity
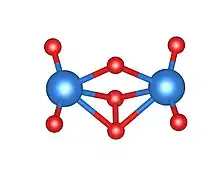
Due to the amorphous nature of am-U2O7, the long-range atomic structure of this compound has not been determined. However, recent computational investigations, chiefly accomplished using density functional theory (DFT), have helped to predict a local structure.[2][4] Resembling a regular uranate compound, two uranyl ([UO2]2+) groups are bridged by a μ2-O atom, where both uranium atoms are bonded to a O-O peroxo unit. In this case, a tetrameric ring would be the most stable conformation of the compound. The presence of a peroxide bond in species obtained in this temperature range is unusual; uranyl peroxide has previously been considered to be the only peroxide bearing uranium compound.[5] Developments on this structure propose a two-site metastudtite and UO3-like bonding environment, including the bond types already mentioned.[4] Few other suggestions for the local atomic structure of am-U2O7 have been made, although a crystalline form of U2O7, calculated as a two-site 6 and 8-coordinate structure, has been reported.[3] In the same study, it was again found that the U2O7 species contained peroxide bonding. Am-U2O7 is known to undergo hydrolysis in the presence of water, to produce a crystalline metaschoepite powder. In addition to a change in crystallinity, this reaction involves a change in colour from orange to bright yellow.
References
- ↑ L. Sweet, C. Henager, S. Hu, T. Johnson, D. Meier, S. Peper and J. Schwantes, PNNL-20951: Investigation of Uranium Polymorphs, PNNL for U.S Department of Energy, 2011. doi:10.13140/RG.2.1.3073.0004
- 1 2 S. Odoh, J. Shamblin, C. Colla, S. Hickam, H. Lobeck, R. Lopez, T. Olds, J. Szymanowski, G. Sigmon, J. Neuefeind, W. Casey, M. Lang, L. Gagliardi and P. Burns, Structure and Reactivity of X-ray Amorphous Uranyl Peroxide, U2O7, J. Inorg. Chem., 2016, 55, pp.3541-3546. doi:10.1021/acs.inorgchem.6b00017
- 1 2 A. Shields, A. Miskowiec, J. Niedziela, M. Kirkegaard, K. Maheshwari, M. Ambrogio, R. Kapsimalis and B. Anderson, Shining a light on amorphous U2O7: A computational approach to understanding amorphous uranium materials, Opt. Mater., 2019, 89, 295-298. doi:10.1016/j.optmat.2019.01.040
- 1 2 Thompson, Nathan B. A.; Middleburgh, Simon C.; Evitts, Lee J.; Gilbert, Matthew R.; Stennett, Martin C.; Hyatt, Neil C. (2020-12-15). "Short communication on further elucidating the structure of amorphous U2O7 by extended X-ray absorption spectroscopy and DFT simulations" (PDF). Journal of Nuclear Materials. 542: 152476. Bibcode:2020JNuM..54252476T. doi:10.1016/j.jnucmat.2020.152476. ISSN 0022-3115. S2CID 225232737.
- ↑ P. Burns and K. Hughes, Studtite, [(UO2)(O2)(H2O)2](H2O)2: The first structure of a peroxide mineral, Am. Mineral., 2003, 88, 1165-1168. doi:10.2138/am-2003-0725