 | |
| Names | |
|---|---|
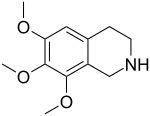
| Preferred IUPAC name
6,7,8-Trimethoxy-1,2,3,4-tetrahydroisoquinoline | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H17NO3 | |
| Molar mass | 223.272 g·mol−1 |
| Melting point | 60–61 °C (140–142 °F; 333–334 K)[1] |
| Boiling point | 144–145 °C (291–293 °F; 417–418 K)[1] at 0.1 Torr |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Anhalinine is a naturally occurring alkaloid[2] which can be isolated from Lophophora williamsii. It is structurally related to mescaline.
See also
References
- 1 2 Taylor, E. P. (1952). "236. Synthetic neuromuscular blocking agents. Part III. Miscellaneous quaternary ammonium salts". Journal of the Chemical Society (Resumed): 1309. doi:10.1039/jr9520001309.
- ↑ Ghansah, E.; Kopsombut, P.; Maleque, M.A.; Brossi, A. (February 1993). "Effects of mescaline and some of its analogs on cholinergic neuromuscular transmission". Neuropharmacology. 32 (2): 169–174. doi:10.1016/0028-3908(93)90097-M.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.