Arp2/3 complex (Actin Related Protein 2/3 complex) is a seven-subunit protein complex that plays a major role in the regulation of the actin cytoskeleton. It is a major component of the actin cytoskeleton and is found in most actin cytoskeleton-containing eukaryotic cells.[2] Two of its subunits, the Actin-Related Proteins ARP2 and ARP3, closely resemble the structure of monomeric actin and serve as nucleation sites for new actin filaments. The complex binds to the sides of existing ("mother") filaments and initiates growth of a new ("daughter") filament at a distinctive 70 degree angle from the mother. Branched actin networks are created as a result of this nucleation of new filaments. The regulation of rearrangements of the actin cytoskeleton is important for processes like cell locomotion, phagocytosis, and intracellular motility of lipid vesicles.
The Arp2/3 complex was named after it was identified in 1994 by affinity chromatography from Acanthamoeba castellanii,[3] though it had been previously isolated in 1989 in a search for proteins that bind to actin filaments in Drosophila melanogaster embryos.[4] It is found in most eukaryotic organisms, but absent from a number of Chromalveolates and plants.[2]
Mechanisms of actin polymerization by Arp2/3
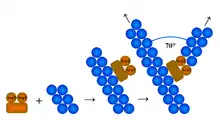
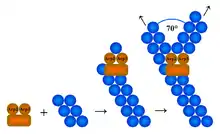
Many actin-related molecules create a free barbed end for polymerization by uncapping or severing pre-existing filaments and using these as actin nucleation cores. However, the Arp2/3 complex stimulates actin polymerization by creating a new nucleation core. Actin nucleation is an initial step in the formation of an actin filament. The nucleation core activity of Arp2/3 is activated by Nucleation Promoting Factors (NPFs) including members of the Wiskott-Aldrich syndrome family protein (WASP, N-WASP, WAVE, and WASH proteins). The V domain of a WASP protein interacts with actin monomers while the CA region associates with the Arp2/3 complex to create a nucleation core. However, de novo nucleation followed by polymerization is not sufficient to form integrated actin networks, since these newly synthesized polymers would not be associated with pre-existing filaments. Thus, the Arp2/3 complex binds to pre-existing filaments so that the new filaments can grow on the old ones and form a functional actin cytoskeleton.[5] Capping proteins limit actin polymerization to the region activated by the Arp2/3 complex, and the elongated filament ends are recapped to prevent depolymerization and thus conserve the actin filament.[6]
The Arp2/3 complex simultaneously controls nucleation of actin polymerization and branching of filaments. Moreover, autocatalysis is observed during Arp2/3-mediated actin polymerization. In this process, the newly formed filaments activate other Arp2/3 complexes, facilitating the formation of branched filaments.
The mechanism of actin filament initiation by Arp2/3 has been disputed. The question is where the complex binds the filament and nucleates a "daughter" filament. Historically two models have been proposed. Recent results favour the side branching model, in which the Arp2/3 complex binds to the side of a pre-existing ("mother") filament at a point different from the nucleation site. Although the field lacks a high-resolution crystal structure, data from electron microscopy,[7][8][9] together with biochemical data on the filament nucleation and capping mechanisms of the Arp2/3 complex,[10] favour side branching. In the alternative barbed end branching model, Arp2/3 only associates at the barbed end of growing filaments, allowing for the elongation of the original filament and the formation of a branched filament.,[11] a model based on kinetic analysis and optical microscopy.
Recent computer docking, independently confirmed by EM data, favors a side-branching model. ARPC2 and ARPC4 together form an area that attach the base of the branch to the side of a mother filament.[12] Large conformational changes occur on nucleotide and WASP binding.[9][13]
Cellular uses of Arp2/3
The Arp2/3 complex appears to be important in a variety of specialized cell functions that involve the actin cytoskeleton. The complex is found in cellular regions characterized by dynamic actin filament activity: in macropinocytic cups, in the leading edge of motile cells (lamellipodia), and in motile actin patches in yeast.[14] In mammals and the social amoeba Dictyostelium discoideum[15][16] it is required for phagocytosis. The complex has also been shown to be involved in the establishment of cell polarity and the migration of fibroblast monolayers in a wound-healing model.[17] In mammalian oocytes, the Arp2/3 complex is involved in oocyte asymmetric division and polar body emission, which result from the failure of spindle migration (a unique feature of oocyte division) and cytokinesis.[18] Moreover, enteropathogenic organisms like Listeria monocytogenes and Shigella use the Arp2/3 complex for actin-polymerization- dependent rocketing movements.[19] The Arp2/3 complex also regulates the intracellular motility of endosomes, lysosomes, pinocytic vesicles, and mitochondria.[20] Moreover, recent studies show that the Arp2/3 complex is essential for proper polar cell expansion in plants. Arp2/3 mutations in Arabidopsis thaliana result in abnormal filament organization, which in turn affects the expansion of trichomes, pavement cells, hypocotyl cells, and root hair cells.[21][22] Chemical inhibition or genetic mutation of the Chlamydomonas reinhardtii Arp2/3 complex decreases the length of flagella.[23][24]
Subunits
The Arp2/3 complex is composed of seven subunits: Arp2/ACTR2, Arp3/ACTR3, p41/ARPC1A&B/Arc40/Sop2/p40, p34/ARPC2/ARC35/p35, p21/ARPC3/ARC18/p19, p20/ARPC4/ARC19/p18, p16/ARPC5/ARC15/p14.[25][26] The subunits Arp2 and Arp3 closely resemble monomeric actin allowing for a thermodynamically stable actin-like dimer. p41 has been proposed to interact with nucleation promoting factors (NPFs) because it is only known to have minor contacts with the mother filament and there is a major loss of nucleation efficiency in the absence of p41. p34 and p20 dimerize to form a structural backbone that mediates the interaction with the mother filament. p21 forms a bridge between Arp3 and the mother filament, increasing nucleation efficiency. p16 tethers Arp2 to the rest of the complex.[27]
References
- ↑ Robinson RC, Turbedsky K, Kaiser DA, Marchand JB, Higgs HN, Choe S, Pollard TD (November 2001). "Crystal structure of Arp2/3 complex". Science. 294 (5547): 1679–84. Bibcode:2001Sci...294.1679R. doi:10.1126/science.1066333. PMID 11721045. S2CID 18088124.
- 1 2 Veltman DM, Insall RH (August 2010). "WASP family proteins: their evolution and its physiological implications". Molecular Biology of the Cell. 21 (16): 2880–93. doi:10.1091/mbc.E10-04-0372. PMC 2921111. PMID 20573979.
- ↑ Machesky LM, Atkinson SJ, Ampe C, Vandekerckhove J, Pollard TD (October 1994). "Purification of a cortical complex containing two unconventional actins from Acanthamoeba by affinity chromatography on profilin-agarose". The Journal of Cell Biology. 127 (1): 107–15. doi:10.1083/jcb.127.1.107. PMC 2120189. PMID 7929556.
- ↑ Miller KG, Field CM, Alberts BM (December 1989). "Actin-binding proteins from Drosophila embryos: a complex network of interacting proteins detected by F-actin affinity chromatography". The Journal of Cell Biology. 109 (6 Pt 1): 2963–75. doi:10.1083/jcb.109.6.2963. PMC 2115944. PMID 2512303.
- ↑ Pollard TD (2007). "Regulation of actin filament assembly by Arp2/3 complex and formins". Annual Review of Biophysics and Biomolecular Structure. 36: 451–77. doi:10.1146/annurev.biophys.35.040405.101936. PMID 17477841.
- ↑ Aguda AH, Burtnick LD, Robinson RC (March 2005). "The state of the filament". EMBO Reports. 6 (3): 220–6. doi:10.1038/sj.embor.7400363. PMC 1299273. PMID 15741975.
- ↑ Egile C, Rouiller I, Xu XP, Volkmann N, Li R, Hanein D (November 2005). "Mechanism of filament nucleation and branch stability revealed by the structure of the Arp2/3 complex at actin branch junctions". PLOS Biology. 3 (11): e383. doi:10.1371/journal.pbio.0030383. PMC 1278936. PMID 16262445.
- ↑ Volkmann N, Amann KJ, Stoilova-McPhie S, Egile C, Winter DC, Hazelwood L, Heuser JE, Li R, Pollard TD, Hanein D (September 2001). "Structure of Arp2/3 complex in its activated state and in actin filament branch junctions". Science. 293 (5539): 2456–9. Bibcode:2001Sci...293.2456V. doi:10.1126/science.1063025. PMID 11533442. S2CID 17427295.
- 1 2 Rouiller I, Xu XP, Amann KJ, Egile C, Nickell S, Nicastro D, Li R, Pollard TD, Volkmann N, Hanein D (March 2008). "The structural basis of actin filament branching by the Arp2/3 complex". The Journal of Cell Biology. 180 (5): 887–95. doi:10.1083/jcb.200709092. PMC 2265399. PMID 18316411.
- ↑ Dayel MJ, Mullins RD (April 2004). "Activation of Arp2/3 complex: addition of the first subunit of the new filament by a WASP protein triggers rapid ATP hydrolysis on Arp2". PLOS Biology. 2 (4): E91. doi:10.1371/journal.pbio.0020091. PMC 387265. PMID 15094799.
- ↑ Pantaloni D, Boujemaa R, Didry D, Gounon P, Carlier MF (July 2000). "The Arp2/3 complex branches filament barbed ends: functional antagonism with capping proteins". Nature Cell Biology. 2 (7): 385–91. doi:10.1038/35017011. PMID 10878802. S2CID 28209883.
- ↑ Goley, ED; Rammohan, A; Znameroski, EA; Firat-Karalar, EN; Sept, D; Welch, MD (4 May 2010). "An actin-filament-binding interface on the Arp2/3 complex is critical for nucleation and branch stability". Proceedings of the National Academy of Sciences of the United States of America. 107 (18): 8159–64. Bibcode:2010PNAS..107.8159G. doi:10.1073/pnas.0911668107. PMC 2889539. PMID 20404198.
- ↑ Padrick, S. B.; Doolittle, L. K.; Brautigam, C. A.; King, D. S.; Rosen, M. K. (16 August 2011). "Arp2/3 complex is bound and activated by two WASP proteins". Proceedings of the National Academy of Sciences. 108 (33): E472–E479. doi:10.1073/pnas.1100236108. ISSN 0027-8424. PMC 3158169. PMID 21676863.
- ↑ Warren DT, Andrews PD, Gourlay CW, Ayscough KR (April 2002). "Sla1p couples the yeast endocytic machinery to proteins regulating actin dynamics". Journal of Cell Science. 115 (Pt 8): 1703–15. doi:10.1242/jcs.115.8.1703. PMID 11950888.
- ↑ May RC, Caron E, Hall A, Machesky LM (April 2000). "Involvement of the Arp2/3 complex in phagocytosis mediated by FcgammaR or CR3". Nature Cell Biology. 2 (4): 246–8. doi:10.1038/35008673. PMID 10783245. S2CID 33742268.
- ↑ Insall R, Müller-Taubenberger A, Machesky L, Köhler J, Simmeth E, Atkinson SJ, Weber I, Gerisch G (November 2001). "Dynamics of the Dictyostelium Arp2/3 complex in endocytosis, cytokinesis, and chemotaxis". Cell Motility and the Cytoskeleton. 50 (3): 115–28. doi:10.1002/cm.10005. PMID 11807934.
- ↑ Magdalena J, Millard TH, Etienne-Manneville S, Launay S, Warwick HK, Machesky LM (February 2003). "Involvement of the Arp2/3 complex and Scar2 in Golgi polarity in scratch wound models". Molecular Biology of the Cell. 14 (2): 670–84. doi:10.1091/mbc.E02-06-0345. PMC 150000. PMID 12589062.
- ↑ Yi K, Unruh JR, Deng M, Slaughter BD, Rubinstein B, Li R (August 2011). "Dynamic maintenance of asymmetric meiotic spindle position through Arp2/3-complex-driven cytoplasmic streaming in mouse oocytes". Nature Cell Biology. 13 (10): 1252–8. doi:10.1038/ncb2320. PMC 3523671. PMID 21874009.
- ↑ Cossart P (June 2000). "Actin-based motility of pathogens: the Arp2/3 complex is a central player". Cellular Microbiology. 2 (3): 195–205. doi:10.1046/j.1462-5822.2000.00053.x. PMID 11207576. S2CID 44343534.
- ↑ Mathur J (April 2005). "The ARP2/3 complex: giving plant cells a leading edge". BioEssays. 27 (4): 377–87. doi:10.1002/bies.20206. PMID 15770684. S2CID 423293.
- ↑ Bannigan A, Baskin TI (December 2005). "Directional cell expansion--turning toward actin". Current Opinion in Plant Biology. 8 (6): 619–24. doi:10.1016/j.pbi.2005.09.002. PMID 16181803.
- ↑ Xu J, Scheres B (December 2005). "Cell polarity: ROPing the ends together". Current Opinion in Plant Biology. 8 (6): 613–8. doi:10.1016/j.pbi.2005.09.003. hdl:1874/21120. PMID 16182602. S2CID 23098801.
- ↑ Avasthi, Prachee; Onishi, Masayuki; Karpiak, Joel; Yamamoto, Ryosuke; Mackinder, Luke; Jonikas, Martin C.; Sale, Winfield S.; Shoichet, Brian; Pringle, John R.; Marshall, Wallace F. (September 2014). "Actin Is Required for IFT Regulation in Chlamydomonas reinhardtii". Current Biology. 24 (17): 2025–2032. doi:10.1016/j.cub.2014.07.038. ISSN 0960-9822. PMC 4160380. PMID 25155506.
- ↑ Bigge, Brae M.; Rosenthal, Nicholas E.; Sept, David; Schroeder, Courtney M.; Avasthi, Prachee (2020-11-24). "Initial ciliary assembly in Chlamydomonas requires Arp2/3-dependent recruitment from a ciliary protein reservoir in the plasma membrane". bioRxiv: 2020.11.24.396002. doi:10.1101/2020.11.24.396002. S2CID 233178920.
- ↑ Goley ED, Welch MD (October 2006). "The ARP2/3 complex: an actin nucleator comes of age". Nature Reviews Molecular Cell Biology. 7 (10): 713–26. doi:10.1038/nrm2026. PMID 16990851. S2CID 20645116.
- ↑ Pizarro-Cerdá, Javier; Chorev, Dror Shlomo; Geiger, Benjamin; Cossart, Pascale (February 2017). "The Diverse Family of Arp2/3 Complexes". Trends in Cell Biology. 27 (2): 93–100. doi:10.1016/j.tcb.2016.08.001. ISSN 0962-8924. PMC 7098815. PMID 27595492.
- ↑ Pizarro-Cerdá J, Chorev DS, Geiger B, Cossart P (February 2017). "The Diverse Family of Arp2/3 Complexes" (PDF). Trends in Cell Biology. 27 (2): 93–100. doi:10.1016/j.tcb.2016.08.001. PMC 7098815. PMID 27595492.
External links
- MBInfo - Arp2/3 Mediated Nucleation
- Arp2-3+Complex at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Arp2/3 filament nucleation model, illustrated with 2D animations and 3D structure-based movies
