| Aspergillus tubingensis | |
|---|---|
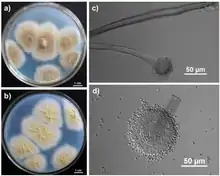 | |
| (a) Colonies growing in Czapek medium for 7 days; (b) The yellowish colonies observed from the reverse side of the Czapek’s agar; (c) Sporophore and spherical sporangium; (d) Conidia and sporangium with bilayer structure | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Fungi |
| Division: | Ascomycota |
| Class: | Eurotiomycetes |
| Order: | Eurotiales |
| Family: | Aspergillaceae |
| Genus: | Aspergillus |
| Species: | A. tubingensis |
| Binomial name | |
| Aspergillus tubingensis R.Mosseray 1934 | |
| Synonyms | |
| |
Aspergillus tubingensis is a darkly pigmented species of fungus in the genus Aspergillus section Nigri.[1][2] It is often confused with Aspergillus niger due to their similar morphology and habitat.[1] A. tubingensis is often involved in food spoilage of fruits and wheat, and industrial fermentation. This species is a rare agent of opportunistic infection.[3]
Background
Aspergillus tubingensis was first discovered by Raoul Mosseray in 1934.[4] The conidia are heavily roughened, 3-5 µm in diameter.[5][6] Whitish to pink sclerotia ranging from 0.5 to 0.8 mm in diameter are often produced. A. tubingensis exists exclusively as an asexual fungus but is understood to be phylogenetically closely related to the other so-called black Aspergilli and sexual states in the genus Petromyces. The production of Ochratoxin A (OTA) was previously thought to be a variable character dependent on strain;[7] however, the production of OTA is thought to be a consistent feature with prior reports of variation arising from the inclusion of misidentified strains (e.g., A. niger) or inconsistencies in test conditions such as incubation time, temperature, and growth medium.[8][9] Other extrolites produced by this fungus include: asperazine, pyranoigrin A, pyrophen, funalenone, and kotanins.[7] When cultured on creatine sucrose agar (CREA) culture medium, A. tubingensis demonstrates good acid production (strong yellow colour change) and a moderate growth rate.[6] A. tubingensis and A. niger have similar morphology and are difficult to distinguish without resorting to more advanced methods. One rapid test that is useful in distinguishing the two taxa, the Ehrlich reaction, queries the presence of indole. In this test, A. tubingensis is negative in contrast to A. niger which produces a positive result. Sequences of protein coding genes such as Calmodulin and β-tubulin also reliable differentiate the two taxa.[10][11] The production of asperazine by A. tubingensis also separates this species from other morphologically similar Aspergilli.[7]
Habitat and ecology
Aspergillus tubingensis exhibits high resistance to ultraviolet light and can grow in elevated temperatures[12] between 30–37 °C (86–99 °F),[13] with optimal growth between 21–36 °C (70–97 °F).[7] In the temperature range of 15–20 °C (59–68 °F), this species is able to produce the mycotoxin, ochratoxin A (OTA).[13] The fungus is tolerant of low pH and has a preference for environments of relatively low water activity.[12] Originally recognized from Chiang Mai, Thailand and China,[7] A. tubingensis is found worldwide in warm climate regions. It is often seen in indoor environments of Croatia and Turkey, with some appearances in the Netherlands, Hungary, Thailand, and Algeria.[14] This species is commonly isolated from soil and plant debris as well as agricultural crops such as grapes, cocoa, coffee, and cereal,[7] and as an agent of rot on apples, grapes, and cereals.[15]
Commercial uses
Because of the paucity of mycotoxin production by A. tubingensis, it has been explored for use in biotechnology and industrial applications.[16] A. tubingensis is generally recognized as safe (GRAS) by the American food and drug administration (FDA).[1] This species is notable for the production enzymes such amylase, lipase, glucose oxidase, phytase, xylanase, acid phosphatase and xylosidase production. Amylase produced by A. tubingensis has potential use in the manufacture of bioethanol from distilled waste water and molasses residues.[17] The fungus is also able to produce commercially scalable organic acids including citric acid, ascorbic acid, and wood preservatives.[16] It is also capable of degrading polyurethane.[18]
In commercial baking, the use of glucose oxidase enzyme (GOD) enhances texture, size, and loaf form. A. tubingensis is part of the microbial consortium involved in the fermentation of Chinese pu'er tea, converting tea polyphenols into bioactive theobromins. [unreliable source?] [19]
In crop production, soil amendation with A. tubingensis has been shown to enhance corn yield through its ability to dissolve phosphates into soil and reduce alkalinity in bauxite residues.[20] The tolerance of A. tubingensis to conditions of high pH enhance its survival in these applications.[20] A. tubingensis has been suggested as a biocontrol agent for the protection of tomato plants against the pathogenic fungus, Fusarium solani.[21] Deleterious effects of this fungus on crop plants are also known. For example, A. tubingensis has been documented in grape vineyards, alongside other black Aspergilli including A. carbonarius and A. niger.[22] In grape production, these Aspergilli have been implicated as important contributors to OTA in grape must.[23]
In 2018, they were investigated for their ability to decompose plastic such as polyurethane in weeks rather than decades.[24]
"The plastic-busting potential was discovered last year by a team of scientists from China and Pakistan, who sought to isolate the fungi that were degrading polyurethane at a waste disposal site in Islamabad. The fungi were identified as aspergillus tubingensis and the scientists observed how it broke down bonds between the different polymers in weeks, rather than the decades it can take plastic to naturally disintegrate."[24]
Opportunistic disease
Fungal keratitis (corneal infection) can be caused by members of the black Aspergilli including A. tubingensis.[25] Aspergillus tubingensis has also been implicated in the infection of maxillary bone following a tooth extraction.[26]
References
- 1 2 3 Oisewacz, Heinz (2002). Industrial Applications. New york: Springer-Verlag Berlin Heidelberg. pp. 264–265.
- ↑ Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.-B.; Hubka, V.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; Varga, J.; Kocsubé, S.; Szigeti, G.; Yaguchi, T.; Frisvad, J.C. (June 2014). "Phylogeny, identification and nomenclature of the genus Aspergillus". Studies in Mycology. 78: 141–173. doi:10.1016/j.simyco.2014.07.004. PMC 4260807. PMID 25492982.
- ↑ Perrone, G; Susca, A; Cozzi, G; Ehrlich, J; Varga, J; Frisvad, JC; Meijer, M; Noonim, P; Mahakarnchanakul, W; Samson, RA (2007). "Biodiversity of Aspergillus species in some important agricultural products". Studies in Mycology. 59: 53–66. doi:10.3114/sim.2007.59.07. PMC 2275197. PMID 18490950.
- ↑ Mosseray, R (1934). "Les Aspergillus de la section Niger Thom et Church". La Cellule. 43: 203–285.
- ↑ Machida, Masayuki; Gomi, Katsuya (2010). Aspergillus: Molecular Biology and Genomics. pp. 28–29.
- 1 2 Silva, D; Batista, L; Rezende, E; Fungaro, M; Sartori, D; Alves, E (2011). "Identification of fungi of the genus Aspergillus section nigri using polyphasic taxonomy". Brazilian Journal of Microbiology. 42 (2): 761–773. doi:10.1590/S1517-838220110002000044 (inactive 1 August 2023). PMC 3769849. PMID 24031691.
{{cite journal}}: CS1 maint: DOI inactive as of August 2023 (link) - 1 2 3 4 5 6 Samson, RA; Noonim, P; Meijer, M; Houbraken, JC; Frisvad, J; Varga, J (2007). "Diagnostic tools to identify black aspergilli". Studies in Mycology. 59: 129–145. doi:10.3114/sim.2007.59.13. PMC 2275192. PMID 18490945.
- ↑ Medina, A; Mateo, R; Lopez-Ocana, L; Valle-Algarra, F; Jimenez, M (2005). "Study of Spanish Grape Mycobiota and Ochratoxin A Production by Isolates of Aspergillus tubingensis and Other Members of Aspergillus Section Nigri". Applied and Environmental Microbiology. 71 (8): 4696–4702. Bibcode:2005ApEnM..71.4696M. doi:10.1128/AEM.71.8.4696-4702.2005. hdl:10550/31430. PMC 1183270. PMID 16085865.
- ↑ Susca, A; Moretti, A; Stea, G; Villani, A; Haidukowski, M; Logrieco, A; Munkvold, G (2014). "Comparison of species composition and fumonisin production in Aspergillus section Nigri populations in maize kernels from USA and Italy". International Journal of Food Microbiology. 188: 75–82. doi:10.1016/j.ijfoodmicro.2014.06.031. PMID 25087207.
- ↑ Kozakiewicz, Z (1989). "Aspergillus species on the stored products". Mycological Papers. 161: 1–188.
- ↑ Bennett, JW (2010). "An overview of genus Aspergillus". In Masayuki Machida; Katsuya Gomi (eds.). Aspergillus: Molecular Biology and Genomics. Horizon Scientific Press. ISBN 978-1-904455-53-0.
- 1 2 World Health Organization (2008). Safety evaluation of certain food additives and contaminants. Geneva.
{{cite book}}: CS1 maint: location missing publisher (link) - 1 2 Botana, L; Sainz, M (2015). Climate change and mycotoxins. Berlin, Boston: Walter de Gruyter.
- ↑ Varga, J; Kocsube, S; Szigeti, G; Baranyi, N; Vagvolgyi, C; Despot, D; Magyar, D; Meijer, M; Samson, R; Klaric, M (2014). "Occurrence of black Aspergilli in indoor environments of six countries". Archives of Industrial Hygiene and Toxicology. 65 (2): 219–223. doi:10.2478/10004-1254-65-2014-2450. PMID 24778343.
- ↑ Anderson, B; Thrane, U (2006). "Food-borne fungi in fruit and cereals and their production of mycotoxins". Advances in Food Mycology. Advances in Experimental Medicine and Biology. Vol. 571. pp. 137–152. doi:10.1007/0-387-28391-9_8. ISBN 978-0-387-28385-2. PMID 16408598.
- 1 2 Olarte, RA; Horn, BW; Singh, R; Carbone, I (2015). "Sexual recombination in Aspergillus tubingensis". Mycologia. 107 (2): 307–312. doi:10.3852/14-233. PMID 25572097. S2CID 42845053.
- ↑ Watanabe, T; Tanaka, M; Masaki, K; Fujii, T; Lefuji, H (2009). "Decolorization and semi-batch continuous treatment of molasses distillery wastewater by Aspergillus tubingensis DCT6". Water Science & Technology. 59 (11): 2179–2185. doi:10.2166/wst.2009.240. PMID 19494457.
- ↑ Khan, Sehroon; Nadir, Sadia; Shah, Zia Ullah; Shah, Aamer Ali; Karunarathna, Samantha C.; Xu, Jianchu; Khan, Afsar; Munir, Shahzad; Hasan, Fariha (June 2017). "Biodegradation of polyester polyurethane by Aspergillus tubingensis". Environmental Pollution. 225: 469–480. doi:10.1016/j.envpol.2017.03.012. PMID 28318785.
- ↑ Wang, Q; Gong, J; Chisti, Y; Sirisansaneeyakul, S (2015). "Fungal isolates from a pu-erh type tea fermentation and their ability to convert tea polyphenols to theabrownin". Journal of Food Science. 80 (4): M809–M817. doi:10.1111/1750-3841.12831. PMID 25799937.
- 1 2 Krishna, P; Reddy, M; Patnaik, S (2005). "Aspergillus tubingensis reduces the pH of the bauxite residue (red mud) amended soils". Water, Air, and Soil Pollution. 167 (1–4): 201–209. Bibcode:2005WASP..167..201K. doi:10.1007/s11270-005-0242-9. S2CID 97808013.
- ↑ Kriaa, Mouna; Mnafgui, Kais; Belhadj, Sahla; El feki, Abdelfattah; Kammoun, Radhouane (2015). "The developmental evaluation of Aspergillus tubingensis CTM 507 glucose oxidase toxicity in Wistar rats". Journal of Food Safety. 35 (2): 263–269. doi:10.1111/jfs.12154.
- ↑ Garcia-cela, E; Crespo-Sempere, A; Ramos, AJ; Sanchis, V; Marin, S (2014). "Ecophysiological characterization of Aspergillus carbonarius, Aspergillus tubingensis and Aspergillus niger isolated from grapes in Spanish vineyards". International Journal of Food Microbiology. 3 (173): 89–98. doi:10.1016/j.ijfoodmicro.2014.06.031. PMID 25087207.
- ↑ Perrone, G; Mulè, G; Susca, A; Battilani, P; Pietri, A; Logrieco, A (2006). "Ochratoxin A production and amplified fragment length polymorphism analysis of Aspergillus carbonarius, Aspergillus tubingensis, and Aspergillus niger strains isolated from grapes in Italy". Applied and Environmental Microbiology. 72 (1): 680–685. Bibcode:2006ApEnM..72..680P. doi:10.1128/AEM.72.1.680-685.2006. PMC 1352186. PMID 16391107.
- 1 2 "Fungi research lifts lid on shy organisms that break down plastic". UN Environment. Retrieved 2018-11-03.
- ↑ Kredics, László; Varga, János; Kocsubé, Sándor; Rajaraman, Revathi; Raghavan, Anita; Dóczi, Ilona; Bhaskar, Madhavan; Németh, Tibor Mihály; Antal, Zsuzsanna; Venkatapathy, Narendran; Vágvölgyi, Csaba; Samson, Robert A; Chockaiya, Manoharan; Palanisamy, Manikandan (2009). "Infectious keratitis caused by Aspergillus tubingensis". Cornea. 28 (8): 951–954. doi:10.1097/ICO.0b013e3181967098. PMID 19654512. S2CID 19506657.
- ↑ Bathoorn, E; Escobar, SN; Sephrkhouy, S; Meijer, M; de Cock, H; Haas, PJ (2013). "Involvement of the opportunistic pathogen Aspergillus tubingensis in osteomyelitis of the maxillary bone: a case report". BMC Infectious Diseases. 13 (1): 59. doi:10.1186/1471-2334-13-59. PMC 3565948. PMID 23374883.
Bibliography
- Russell, Jonathan R, Huang, Jeffrey, Anand, Pria, Kucera, Kaury, Sandoval, Amanda G, Dantzler, Kathleen W, Hickman, DaShawn, Jee, Justin, Kimovec, Farrah M, Koppstein, David, Marks, Daniel H, Mittermiller, Paul A, Núñez, Salvador Joel, Santiago, Marina, Townes, Maria A, Vishnevetsky, Michael, Williams, Neely E, Vargas, Mario Percy Núñez, Boulanger, Lori-Ann, Bascom-Slack, Carol, and Strobel, Scott A. "Biodegradation of Polyester Polyurethane by Endophytic Fungi." Applied and Environmental Microbiology 77.17 (2011): 6076–084. Web.
- Álvarez-Barragán, Joyce, Domínguez-Malfavón, Lilianha, Vargas-Suárez, Martín, González-Hernández, Ricardo, Aguilar-Osorio, Guillermo, and Loza-Tavera, Herminia. "Biodegradative Activities of Selected Environmental Fungi on a Polyester Polyurethane Varnish and Polyether Polyurethane Foams." Applied and Environmental Microbiology 82.17 (2016): 5225–235. Web.
- Gilbert, Marianne. Brydsons Plastics Materials. Butterworth-Heinemann Is an Imprint of Elsevier, 2017.