Autophagy (or autophagocytosis; from the Ancient Greek αὐτόφαγος, autóphagos, meaning "self-devouring"[1] and κύτος, kýtos, meaning "hollow")[2] is the natural, conserved degradation of the cell that removes unnecessary or dysfunctional components through a lysosome-dependent regulated mechanism.[3] It allows the orderly degradation and recycling of cellular components.[4][5] Although initially characterized as a primordial degradation pathway induced to protect against starvation, it has become increasingly clear that autophagy also plays a major role in the homeostasis of non-starved cells.[6] Defects in autophagy have been linked to various human diseases, including neurodegeneration and cancer, and interest in modulating autophagy as a potential treatment for these diseases has grown rapidly.[6][7]
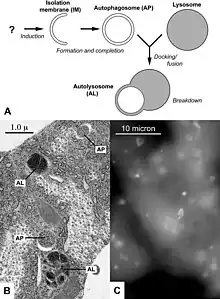
Four forms of autophagy have been identified: macroautophagy, microautophagy, chaperone-mediated autophagy (CMA), and crinophagy.[8][9][10] In macroautophagy (the most thoroughly researched form of autophagy), cytoplasmic components (like mitochondria) are targeted and isolated from the rest of the cell within a double-membrane vesicle known as an autophagosome,[11][12] which, in time, fuses with an available lysosome, bringing its specialty process of waste management and disposal; and eventually the contents of the vesicle (now called an autolysosome) are degraded and recycled. In crinophagy (the least well-known and researched form of autophagy), unnecessary secretory granules are degraded and recycled.[8]
In disease, autophagy has been seen as an adaptive response to stress, promoting survival of the cell; but in other cases, it appears to promote cell death and morbidity. In the extreme case of starvation, the breakdown of cellular components promotes cellular survival by maintaining cellular energy levels.
The word "autophagy" was in existence and frequently used from the middle of the 19th century.[13] In its present usage, the term autophagy was coined by Belgian biochemist Christian de Duve in 1963 based on his discovery of the functions of lysosome.[3] The identification of autophagy-related genes in yeast in the 1990s allowed researchers to deduce the mechanisms of autophagy,[14][15][16][17][18] which eventually led to the award of the 2016 Nobel Prize in Physiology or Medicine to Japanese researcher Yoshinori Ohsumi.[19]
History
Autophagy was first observed by Keith R. Porter and his student Thomas Ashford at the Rockefeller Institute. In January 1962 they reported an increased number of lysosomes in rat liver cells after the addition of glucagon, and that some displaced lysosomes towards the centre of the cell contained other cell organelles such as mitochondria. They called this autolysis after Christian de Duve and Alex B. Novikoff. However Porter and Ashford wrongly interpreted their data as lysosome formation (ignoring the pre-existing organelles). Lysosomes could not be cell organelles, but part of cytoplasm such as mitochondria, and that hydrolytic enzymes were produced by microbodies.[20] In 1963 Hruban, Spargo and colleagues published a detailed ultrastructural description of "focal cytoplasmic degradation", which referenced a 1955 German study of injury-induced sequestration. Hruban, Spargo and colleagues recognized three continuous stages of maturation of the sequestered cytoplasm to lysosomes, and that the process was not limited to injury states that functioned under physiological conditions for "reutilization of cellular materials", and the "disposal of organelles" during differentiation.[21] Inspired by this discovery, de Duve christened the phenomena "autophagy". Unlike Porter and Ashford, de Duve conceived the term as a part of lysosomal function while describing the role of glucagon as a major inducer of cell degradation in the liver. With his student Russell Deter, he established that lysosomes are responsible for glucagon-induced autophagy.[22][23] This was the first time the fact that lysosomes are the sites of intracellular autophagy was established.[3][24][25]
In the 1990s several groups of scientists independently discovered autophagy-related genes using the budding yeast. Notably, Yoshinori Ohsumi and Michael Thumm examined starvation-induced non-selective autophagy;[15][16][17] in the meantime, Daniel J. Klionsky discovered the cytoplasm-to-vacuole targeting (CVT) pathway, which is a form of selective autophagy.[14][18] They soon found that they were in fact looking at essentially the same pathway, just from different angles.[26][27] Initially, the genes discovered by these and other yeast groups were given different names (APG, AUT, CVT, GSA, PAG, PAZ, and PDD). A unified nomenclature was advocated in 2003 by the yeast researchers to use ATG to denote autophagy genes.[28] The 2016 Nobel Prize in Physiology or Medicine was awarded to Yoshinori Ohsumi,[19] although some have pointed out that the award could have been more inclusive.[29]
The field of autophagy research experienced accelerated growth at the turn of the 21st century. Knowledge of ATG genes provided scientists more convenient tools to dissect functions of autophagy in human health and disease. In 1999, a landmark discovery connecting autophagy with cancer was published by Beth Levine's group.[30] To this date, relationship between cancer and autophagy continues to be a main theme of autophagy research. The roles of autophagy in neurodegeneration and immune defense also received considerable attention. In 2003, the first Gordon Research Conference on autophagy was held at Waterville.[31] In 2005, Daniel J Klionsky launched Autophagy, a scientific journal dedicated to this field. The first Keystone Symposia on autophagy was held in 2007 at Monterey.[32] In 2008, Carol A Mercer created a BHMT fusion protein (GST-BHMT), which showed starvation-induced site-specific fragmentation in cell lines. The degradation of betaine homocysteine methyltransferase (BHMT), a metabolic enzyme, could be used to assess autophagy flux in mammalian cells. Macro, micro, and Chaperone mediated autophagy are mediated by autophagy-related genes and their associated enzymes.[11][12][33][34][35] Macroautophagy is then divided into bulk and selective autophagy. In the selective autophagy is the autophagy of organelles; mitophagy,[36] lipophagy,[37] pexophagy,[38] chlorophagy,[39] ribophagy[40] and others.
Macroautophagy is the main pathway, used primarily to eradicate damaged cell organelles or unused proteins.[41] First the phagophore engulfs the material that needs to be degraded, which forms a double membrane known as an autophagosome, around the organelle marked for destruction.[34][42] The autophagosome then travels through the cytoplasm of the cell to a lysosome in mammals, or vacuoles in yeast and plants,[43] and the two organelles fuse.[34] Within the lysosome/vacuole, the contents of the autophagosome are degraded via acidic lysosomal hydrolase.[44]
Microautophagy, on the other hand, involves the direct engulfment of cytoplasmic material into the lysosome.[45] This occurs by invagination, meaning the inward folding of the lysosomal membrane, or cellular protrusion.[42]
Chaperone-mediated autophagy, or CMA, is a very complex and specific pathway, which involves the recognition by the hsc70-containing complex.[42][46] This means that a protein must contain the recognition site for this hsc70 complex which will allow it to bind to this chaperone, forming the CMA- substrate/chaperone complex.[44] This complex then moves to the lysosomal membrane-bound protein that will recognise and bind with the CMA receptor. Upon recognition, the substrate protein gets unfolded and it is translocated across the lysosome membrane with the assistance of the lysosomal hsc70 chaperone.[33][34] CMA is significantly different from other types of autophagy because it translocates protein material in a one by one manner, and it is extremely selective about what material crosses the lysosomal barrier.[41]
Mitophagy is the selective degradation of mitochondria by autophagy. It often occurs to defective mitochondria following damage or stress. Mitophagy promotes the turnover of mitochondria and prevents the accumulation of dysfunctional mitochondria which can lead to cellular degeneration. It is mediated by Atg32 (in yeast) and NIX and its regulator BNIP3 in mammals. Mitophagy is regulated by PINK1 and parkin proteins. The occurrence of mitophagy is not limited to the damaged mitochondria but also involves undamaged ones.[35]
Lipophagy is the degradation of lipids by autophagy,[37] a function which has been shown to exist in both animal and fungal cells.[47] The role of lipophagy in plant cells, however, remains elusive.[48] In lipophagy the target are lipid structures called lipid droplets (LDs), spheric "organelles" with a core of mainly triacylglycerols (TAGs) and a unilayer of phospholipids and membrane proteins. In animal cells the main lipophagic pathway is via the engulfment of LDs by the phagophore, macroautophagy. In fungal cells on the other hand microplipophagy constitutes the main pathway and is especially well studied in the budding yeast Saccharomyces cerevisiae[49]. Lipophagy was first discovered in mice and published 2009.[50]
Targeted interplay between bacterial pathogens and host autophagy
Autophagy targets genus-specific proteins, so orthologous proteins which share sequence homology with each other are recognized as substrates by a particular autophagy targeting protein. There exists a complementarity of autophagy targeting proteins which potentially increase infection risk upon mutation. The lack of overlap among the targets of the 3 autophagy proteins and the large overlap in terms of the genera show that autophagy could target different sets of bacterial proteins from a same pathogen. On one hand, the redundancy in targeting a same genera is beneficial for robust pathogen recognition. But, on the other hand, the complementarity in the specific bacterial proteins could make the host more susceptible to chronic disorders and infections if the gene encoding one of the autophagy targeting proteins becomes mutated, and the autophagy system is overloaded or suffers other malfunctions. Moreover, autophagy targets virulence factors and virulence factors responsible for more general functions such as nutrient acquisition and motility are recognized by multiple autophagy targeting proteins. And the specialized virulence factors such as autolysins, and iron sequestering proteins are potentially recognized uniquely by a single autophagy targeting protein. The autophagy proteins CALCOCO2/NDP52 and MAP1LC3/LC3 may have evolved specifically to target pathogens or pathogenic proteins for autophagic degradation. While SQSTM1/p62 targets more generic bacterial proteins containing a target motif but not related to virulence.[51]
On the other hand, bacterial proteins from various pathogenic genera are also able to modulate autophagy. There are genus-specific patterns in the phases of autophagy that are potentially regulated by a given pathogen group. Some autophagy phases can only be modulated by particular pathogens, while some phases are modulated by multiple pathogen genera. Some of the interplay-related bacterial proteins have proteolytic and post-translational activity such as phosphorylation and ubiquitination and can interfere with the activity of autophagy proteins.[51]
Molecular biology
Autophagy is executed by autophagy-related (Atg) genes. Prior to 2003, ten or more names were used, but after this point a unified nomenclature was devised by fungal autophagy researchers.[52] Atg or ATG stands for autophagy related. It does not specify gene or a protein.[52]
The first autophagy genes were identified by genetic screens conducted in Saccharomyces cerevisiae.[14][15][16][17][18] Following their identification those genes were functionally characterized and their orthologs in a variety of different organisms were identified and studied.[11][53] Today, thirty-six Atg proteins have been classified as especially important for autophagy, of which 18 belong to the core machinery[54]
In mammals, amino acid sensing and additional signals such as growth factors and reactive oxygen species regulate the activity of the protein kinases mTOR and AMPK.[53][55] These two kinases regulate autophagy through inhibitory phosphorylation of the Unc-51-like kinases ULK1 and ULK2 (mammalian homologues of Atg1).[56] Induction of autophagy results in the dephosphorylation and activation of the ULK kinases. ULK is part of a protein complex containing Atg13, Atg101 and FIP200. ULK phosphorylates and activates Beclin-1 (mammalian homologue of Atg6),[57] which is also part of a protein complex. The autophagy-inducible Beclin-1 complex[58] contains the proteins PIK3R4(p150), Atg14L and the class III phosphatidylinositol 3-phosphate kinase (PI(3)K) Vps34.[59] The active ULK and Beclin-1 complexes re-localize to the site of autophagosome initiation, the phagophore, where they both contribute to the activation of downstream autophagy components.[60][61]
Once active, VPS34 phosphorylates the lipid phosphatidylinositol to generate phosphatidylinositol 3-phosphate (PtdIns(3)P) on the surface of the phagophore. The generated PtdIns(3)P is used as a docking point for proteins harboring a PtdIns(3)P binding motif. WIPI2, a PtdIns(3)P binding protein of the WIPI (WD-repeat protein interacting with phosphoinositides) protein family, was recently shown to physically bind ATG16L1.[62] Atg16L1 is a member of an E3-like protein complex involved in one of two ubiquitin-like conjugation systems essential for autophagosome formation. The FIP200 cis-Golgi-derived membranes fuse with ATG16L1-positive endosomal membranes to form the prophagophore termed HyPAS (hybrid pre-autophagosomal structure).[63] ATG16L1 binding to WIPI2[64] mediates ATG16L1's activity. This leads to downstream conversion of prophagophore into ATG8-positive phagophore[63] via a ubiquitin-like conjugation system.
The first of the two ubiquitin-like conjugation systems involved in autophagy covalently binds the ubiquitin-like protein Atg12 to Atg5. The resulting conjugate protein then binds ATG16L1 to form an E3-like complex which functions as part of the second ubiquitin-like conjugation system.[65] This complex binds and activates Atg3, which covalently attaches mammalian homologues of the ubiquitin-like yeast protein ATG8 (LC3A-C, GATE16, and GABARAPL1-3), the most studied being LC3 proteins, to the lipid phosphatidylethanolamine (PE) on the surface of autophagosomes.[66] Lipidated LC3 contributes to the closure of autophagosomes,[67] and enables the docking of specific cargos and adaptor proteins such as Sequestosome-1/p62.[68] The completed autophagosome then fuses with a lysosome through the actions of multiple proteins, including SNAREs[69][70] and UVRAG.[71] Following the fusion LC3 is retained on the vesicle's inner side and degraded along with the cargo, while the LC3 molecules attached to the outer side are cleaved off by Atg4 and recycled.[72] The contents of the autolysosome are subsequently degraded and their building blocks are released from the vesicle through the action of permeases.[73]
Sirtuin 1 (SIRT1) stimulates autophagy by preventing acetylation of proteins (via deacetylation) required for autophagy as demonstrated in cultured cells and embryonic and neonatal tissues.[74] This function provides a link between sirtuin expression and the cellular response to limited nutrients due to caloric restriction.[75]
Functions
Nutrient starvation
Autophagy has roles in various cellular functions. One particular example is in yeasts, where the nutrient starvation induces a high level of autophagy. This allows unneeded proteins to be degraded and the amino acids recycled for the synthesis of proteins that are essential for survival.[76][77][78] In higher eukaryotes, autophagy is induced in response to the nutrient depletion that occurs in animals at birth after severing off the trans-placental food supply, as well as that of nutrient starved cultured cells and tissues.[79][80] Mutant yeast cells that have a reduced autophagic capability rapidly perish in nutrition-deficient conditions.[81] Studies on the apg mutants suggest that autophagy via autophagic bodies is indispensable for protein degradation in the vacuoles under starvation conditions, and that at least 15 APG genes are involved in autophagy in yeast.[81] A gene known as ATG7 has been implicated in nutrient-mediated autophagy, as mice studies have shown that starvation-induced autophagy was impaired in atg7-deficient mice.[80]
Infection
Vesicular stomatitis virus is believed to be taken up by the autophagosome from the cytosol and translocated to the endosomes where detection takes place by a pattern recognition receptor called toll-like receptor 7, detecting single stranded RNA. Following activation of the toll-like receptor, intracellular signaling cascades are initiated, leading to induction of interferon and other antiviral cytokines. A subset of viruses and bacteria subvert the autophagic pathway to promote their own replication.[82] Galectin-8 has recently been identified as an intracellular "danger receptor", able to initiate autophagy against intracellular pathogens. When galectin-8 binds to a damaged vacuole, it recruits an autophagy adaptor such as NDP52 leading to the formation of an autophagosome and bacterial degradation.[83]
Repair mechanism
Autophagy degrades damaged organelles, cell membranes and proteins, and insufficient autophagy is thought to be one of the main reasons for the accumulation of damaged cells and aging.[84] Autophagy and autophagy regulators are involved in response to lysosomal damage, often directed by galectins such as galectin-3 and galectin-8.
Programmed cell death
One of the mechanisms of programmed cell death (PCD) is associated with the appearance of autophagosomes and depends on autophagy proteins. This form of cell death most likely corresponds to a process that has been morphologically defined as autophagic PCD. One question that constantly arises, however, is whether autophagic activity in dying cells is the cause of death or is actually an attempt to prevent it. Morphological and histochemical studies have not so far proved a causative relationship between the autophagic process and cell death. In fact, there have recently been strong arguments that autophagic activity in dying cells might actually be a survival mechanism.[85][86] Studies of the metamorphosis of insects have shown cells undergoing a form of PCD that appears distinct from other forms; these have been proposed as examples of autophagic cell death.[87] Recent pharmacological and biochemical studies have proposed that survival and lethal autophagy can be distinguished by the type and degree of regulatory signaling during stress particularly after viral infection.[88] Although promising, these findings have not been examined in non-viral systems.
Exercise
Autophagy is essential for basal homeostasis; it is also extremely important in maintaining muscle homeostasis during physical exercise.[89][90] Autophagy at the molecular level is only partially understood. A study of mice shows that autophagy is important for the ever-changing demands of their nutritional and energy needs, particularly through the metabolic pathways of protein catabolism. In a 2012 study conducted by the University of Texas Southwestern Medical Center in Dallas, mutant mice (with a knock-in mutation of BCL2 phosphorylation sites to produce progeny that showed normal levels of basal autophagy yet were deficient in stress-induced autophagy) were tested to challenge this theory. Results showed that when compared to a control group, these mice illustrated a decrease in endurance and an altered glucose metabolism during acute exercise.[91]
Another study demonstrated that skeletal muscle fibers of collagen VI in knockout mice showed signs of degeneration due to an insufficiency of autophagy which led to an accumulation of damaged mitochondria and excessive cell death.[92] Exercise-induced autophagy was unsuccessful however; but when autophagy was induced artificially post-exercise, the accumulation of damaged organelles in collagen VI deficient muscle fibres was prevented and cellular homeostasis was maintained. Both studies demonstrate that autophagy induction may contribute to the beneficial metabolic effects of exercise and that it is essential in the maintaining of muscle homeostasis during exercise, particularly in collagen VI fibers.[91][90][92]
Work at the Institute for Cell Biology, University of Bonn, showed that a certain type of autophagy, i.e. chaperone-assisted selective autophagy (CASA), is induced in contracting muscles and is required for maintaining the muscle sarcomere under mechanical tension.[93] The CASA chaperone complex recognizes mechanically damaged cytoskeleton components and directs these components through a ubiquitin-dependent autophagic sorting pathway to lysosomes for disposal. This is necessary for maintaining muscle activity.[93][94]
Osteoarthritis
Because autophagy decreases with age and age is a major risk factor for osteoarthritis, the role of autophagy in the development of this disease is suggested. Proteins involved in autophagy are reduced with age in both human and mouse articular cartilage.[95] Mechanical injury to cartilage explants in culture also reduced autophagy proteins.[96] Autophagy is constantly activated in normal cartilage but it is compromised with age and precedes cartilage cell death and structural damage.[97] Thus autophagy is involved in a normal protective process (chondroprotection) in the joint.
Cancer
Cancer often occurs when several different pathways that regulate cell differentiation are disturbed. Autophagy plays an important role in cancer – both in protecting against cancer as well as potentially contributing to the growth of cancer.[85][98] Autophagy can contribute to cancer by promoting survival of tumor cells that have been starved, or that degrade apoptotic mediators through autophagy: in such cases, use of inhibitors of the late stages of autophagy (such as chloroquine), on the cells that use autophagy to survive, increases the number of cancer cells killed by antineoplastic drugs.[99]
The role of autophagy in cancer is one that has been highly researched and reviewed. There is evidence that emphasizes the role of autophagy as both a tumor suppressor and a factor in tumor cell survival. Recent research has shown, however, that autophagy is more likely to be used as a tumor suppressor according to several models.[98]
Tumor suppressor
Several experiments have been done with mice and varying Beclin1, a protein that regulates autophagy. When the Beclin1 gene was altered to be heterozygous (Beclin 1+/-), the mice were found to be tumor-prone.[100] However, when Beclin1 was overexpressed, tumor development was inhibited.[30] Care should be exercised when interpreting phenotypes of beclin mutants and attributing the observations to a defect in autophagy, however: Beclin1 is generally required for phosphatidylinositol 3- phosphate production and as such it affects numerous lysosomal and endosomal functions, including endocytosis and endocytic degradation of activated growth factor receptors. In support of the possibility that Beclin1 affects cancer development through an autophagy-independent pathway is the fact that core autophagy factors which are not known to affect other cellular processes and are definitely not known to affect cell proliferation and cell death, such as Atg7 or Atg5, show a much different phenotype when the respective gene is knocked out, which does not include tumor formation. In addition, full knockout of Beclin1 is embryonic lethal whereas knockout of Atg7 or Atg5 is not.
Necrosis and chronic inflammation also has been shown to be limited through autophagy which helps protect against the formation of tumor cells.[101]
Mechanism of cell death
Cells that undergo an extreme amount of stress experience cell death either through apoptosis or necrosis. Prolonged autophagy activation leads to a high turnover rate of proteins and organelles. A high rate above the survival threshold may kill cancer cells with a high apoptotic threshold.[102][103] This technique can be utilized as a therapeutic cancer treatment.[85]
Tumor cell survival
Alternatively, autophagy has also been shown to play a large role in tumor cell survival. In cancerous cells, autophagy is used as a way to deal with stress on the cell.[104] Induction of autophagy by miRNA-4673, for example, is a pro-survival mechanism that improves the resistance of cancer cells to radiation.[105] Once these autophagy related genes were inhibited, cell death was potentiated.[106] The increase in metabolic energy is offset by autophagy functions. These metabolic stresses include hypoxia, nutrient deprivation, and an increase in proliferation. These stresses activate autophagy in order to recycle ATP and maintain survival of the cancerous cells.[102] Autophagy has been shown to enable continued growth of tumor cells by maintaining cellular energy production. By inhibiting autophagy genes in these tumors cells, regression of the tumor and extended survival of the organs affected by the tumors were found. Furthermore, inhibition of autophagy has also been shown to enhance the effectiveness of anticancer therapies.[102]
Therapeutic target
New developments in research have found that targeted autophagy may be a viable therapeutic solution in fighting cancer. As discussed above, autophagy plays both a role in tumor suppression and tumor cell survival. Thus, the qualities of autophagy can be used as a strategy for cancer prevention. The first strategy is to induce autophagy and enhance its tumor suppression attributes. The second strategy is to inhibit autophagy and thus induce apoptosis.[106]
The first strategy has been tested by looking at dose-response anti-tumor effects during autophagy-induced therapies. These therapies have shown that autophagy increases in a dose-dependent manner. This is directly related to the growth of cancer cells in a dose-dependent manner as well.[104][103] These data support the development of therapies that will encourage autophagy. Secondly, inhibiting the protein pathways directly known to induce autophagy may also serve as an anticancer therapy.[106][103]
The second strategy is based on the idea that autophagy is a protein degradation system used to maintain homeostasis and the findings that inhibition of autophagy often leads to apoptosis. Inhibition of autophagy is riskier as it may lead to cell survival instead of the desired cell death.[104]
Negative regulators of autophagy
Negative regulators of autophagy, such as mTOR, cFLIP, EGFR, (GAPR-1), and Rubicon are orchestrated to function within different stages of the autophagy cascade. The end-products of autophagic digestion may also serve as a negative-feedback regulatory mechanism to stop prolonged activity.[107]
The interface between inflammation and autophagy
Regulators of autophagy control regulators of inflammation, and vice versa.[108] Cells of vertebrate organisms normally activate inflammation to enhance the capacity of the immune system to clear infections and to initiate the processes that restore tissue structure and function.[109] Therefore, it is critical to couple regulation of mechanisms for removal of cellular and bacterial debris to the principal factors that regulate inflammation: The degradation of cellular components by the lysosome during autophagy serves to recycle vital molecules and generate a pool of building blocks to help the cell respond to a changing microenvironment.[110] Proteins that control inflammation and autophagy form a network that is critical for tissue functions, which is dysregulated in cancer: In cancer cells, aberrantly expressed and mutant proteins increase the dependence of cell survival on the “rewired” network of proteolytic systems that protects malignant cells from apoptotic proteins and from recognition by the immune system.[111] This renders cancer cells vulnerable to intervention on regulators of autophagy.
Parkinson's disease
Parkinson's disease is a neurodegenerative disorder partially caused by the cell death of brain and brain stem cells in many nuclei like the substantia nigra. Parkinson's disease is characterized by inclusions of a protein called alpha-synuclien (Lewy bodies) in affected neurons that cells cannot break down. Deregulation of the autophagy pathway and mutation of alleles regulating autophagy are believed to cause neurodegenerative diseases. Autophagy is essential for neuronal survival. Without efficient autophagy, neurons gather ubiquitinated protein aggregates and degrade. Ubiquitinated proteins are proteins that have been tagged with ubiquitin to get degraded. Mutations of synuclein alleles lead to lysosome pH increase and hydrolase inhibition. As a result, lysosomes degradative capacity is decreased. There are several genetic mutations implicated in the disease, including loss of function PINK1[112] and Parkin.[113] Loss of function in these genes can lead to damaged mitochondrial accumulation and protein aggregates that can lead to cellular degeneration. Mitochondria is involved in Parkinson's disease. In idiopathic Parkinson's disease, the disease is commonly caused by dysfunctional mitochondria, cellular oxidative stress, autophagic alterations and the aggregation of proteins. These can lead to mitochondrial swelling and depolarization.[114]
Type 2 diabetes
Excessive activity of the crinophagy form of autophagy in the insulin-producing beta cells of the pancreas could reduce the quantity of insulin available for secretion, leading to type 2 diabetes.[8]
Significance of autophagy as a drug target
Since dysregulation of autophagy is involved in the pathogenesis of a broad range of diseases, great efforts are invested to identify and characterize small synthetic or natural molecules that can regulate it.[115]
See also
- Apoptosis – Programmed cell death in multicellular organisms
- Autophagy database
- Autophagin – Protease
- Mitophagy – autophagic process in which mitochondria are delivered to the vacuole and degraded
- Residual body – vesicles containing indigestible materials, part of lysosomal digestion
- Sub-lethal damage – Damaging changes to a biological cell
References
- ↑ Liddell HG, Scott R, Jone HS. "αὐτό-φαγος". A Greek–English Lexicon. tufts.edu. Retrieved 6 September 2018.
- ↑ Liddell HG, Scott R, Jone HS. "κύτος". A Greek–English Lexicon. tufts.edu. Retrieved 6 September 2018.
- 1 2 3 Klionsky DJ (August 2008). "Autophagy revisited: a conversation with Christian de Duve". Autophagy. 4 (6): 740–3. doi:10.4161/auto.6398. PMID 18567941. S2CID 6198427.
- ↑ Mizushima N, Komatsu M (November 2011). "Autophagy: renovation of cells and tissues". Cell. 147 (4): 728–41. doi:10.1016/j.cell.2011.10.026. PMID 22078875.
- ↑ Kobayashi S (2015). "Choose Delicately and Reuse Adequately: The Newly Revealed Process of Autophagy". Biological & Pharmaceutical Bulletin. 38 (8): 1098–103. doi:10.1248/bpb.b15-00096. PMID 26235572.
- 1 2 Djajadikerta A, Keshri S, Pavel M, Prestil R, Ryan L, Rubinsztein DC (April 2020). "Autophagy Induction as a Therapeutic Strategy for Neurodegenerative Diseases". Journal of Molecular Biology. 432 (8): 2799–2821. doi:10.1016/j.jmb.2019.12.035. PMID 31887286. S2CID 209518157.
- ↑ Levy JM, Towers CG, Thorburn A (September 2017). "Targeting autophagy in cancer". Nature Reviews. Cancer. 17 (9): 528–542. doi:10.1038/nrc.2017.53. PMC 5975367. PMID 28751651.
- 1 2 3 Csizmadia T, Juhász G (2020). "Crinophagy mechanisms and its potential role in human health and disease". Autophagy in health and disease. Progress in Molecular Biology and Translational Science. Vol. 172. pp. 239–255. doi:10.1016/bs.pmbts.2020.02.002. ISBN 9780128220214. PMID 32620244. S2CID 212903191.
- ↑ Parzych, Katherine R.; Klionsky, Daniel J. (2014-01-20). "An Overview of Autophagy: Morphology, Mechanism, and Regulation". Antioxidants & Redox Signaling. 20 (3): 460–473. doi:10.1089/ars.2013.5371. ISSN 1523-0864. PMC 3894687. PMID 23725295.
- ↑ Hill, Rhianna Mae; Fok, Matthew; Grundy, Gabrielle; Parsons, Jason Luke; Rocha, Sonia (2023-10-11). "The role of autophagy in hypoxia-induced radioresistance". Radiotherapy and Oncology. 189: 109951. doi:10.1016/j.radonc.2023.109951. ISSN 0167-8140. PMID 37838322. S2CID 264073704.
- 1 2 3 Mizushima N, Yoshimori T, Ohsumi Y (10 November 2011). "The role of Atg proteins in autophagosome formation". Annual Review of Cell and Developmental Biology. 27 (1): 107–32. doi:10.1146/annurev-cellbio-092910-154005. PMID 21801009.
- 1 2 Xie Z, Klionsky DJ (October 2007). "Autophagosome formation: core machinery and adaptations". Nature Cell Biology. 9 (10): 1102–9. doi:10.1038/ncb1007-1102. PMID 17909521. S2CID 26402002.
- ↑ Ktistakis NT (2017). "In praise of M. Anselmier who first used the term "autophagie" in 1859". Autophagy. 13 (12): 2015–2017. doi:10.1080/15548627.2017.1367473. PMC 5788564. PMID 28837378.
- 1 2 3 Klionsky DJ, Cueva R, Yaver DS (October 1992). "Aminopeptidase I of Saccharomyces cerevisiae is localized to the vacuole independent of the secretory pathway". The Journal of Cell Biology. 119 (2): 287–99. doi:10.1083/jcb.119.2.287. PMC 2289658. PMID 1400574.
- 1 2 3 Takeshige K, Baba M, Tsuboi S, Noda T, Ohsumi Y (October 1992). "Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction". The Journal of Cell Biology. 119 (2): 301–11. doi:10.1083/jcb.119.2.301. PMC 2289660. PMID 1400575.
- 1 2 3 Thumm M, Egner R, Koch B, Schlumpberger M, Straub M, Veenhuis M, Wolf DH (August 1994). "Isolation of autophagocytosis mutants of Saccharomyces cerevisiae". FEBS Letters. 349 (2): 275–80. doi:10.1016/0014-5793(94)00672-5. PMID 8050581. S2CID 26072787.
- 1 2 3 Tsukada M, Ohsumi Y (October 1993). "Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae". FEBS Letters. 333 (1–2): 169–74. doi:10.1016/0014-5793(93)80398-e. PMID 8224160. S2CID 46017791.
- 1 2 3 Harding TM, Morano KA, Scott SV, Klionsky DJ (November 1995). "Isolation and characterization of yeast mutants in the cytoplasm to vacuole protein targeting pathway". The Journal of Cell Biology. 131 (3): 591–602. doi:10.1083/jcb.131.3.591. PMC 2120622. PMID 7593182.
- 1 2 "The Nobel Prize in Physiology or Medicine 2016". The Nobel Foundation. 3 October 2016. Retrieved 3 October 2016.
- ↑ Ashford TP, Porter KR (January 1962). "Cytoplasmic components in hepatic cell lysosomes". The Journal of Cell Biology. 12 (1): 198–202. doi:10.1083/jcb.12.1.198. PMC 2106008. PMID 13862833.
- ↑ Hruban Z, Spargo B, Swift H, Wissler RW, Kleinfeld RG (June 1963). "Focal cytoplasmic degradation". The American Journal of Pathology. 42 (6): 657–83. PMC 1949709. PMID 13955261.
- ↑ Deter RL, Baudhuin P, De Duve C (November 1967). "Participation of lysosomes in cellular autophagy induced in rat liver by glucagon". The Journal of Cell Biology. 35 (2): C11–6. doi:10.1083/jcb.35.2.c11. PMC 2107130. PMID 6055998.
- ↑ Deter RL, De Duve C (May 1967). "Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes". The Journal of Cell Biology. 33 (2): 437–49. doi:10.1083/jcb.33.2.437. PMC 2108350. PMID 4292315.
- ↑ de Duve C (December 1983). "Lysosomes revisited". European Journal of Biochemistry. 137 (3): 391–7. doi:10.1111/j.1432-1033.1983.tb07841.x. PMID 6319122.
- ↑ Dunn WA, Schroder LA, Aris JP (2013). "Historical overview of autophagy". In Wang HG (ed.). Autophagy and Cancer. Springer. pp. 3–4. ISBN 9781461465614.
- ↑ Harding TM, Hefner-Gravink A, Thumm M, Klionsky DJ (July 1996). "Genetic and phenotypic overlap between autophagy and the cytoplasm to vacuole protein targeting pathway". The Journal of Biological Chemistry. 271 (30): 17621–4. doi:10.1074/jbc.271.30.17621. PMID 8663607.
- ↑ Scott SV, Hefner-Gravink A, Morano KA, Noda T, Ohsumi Y, Klionsky DJ (October 1996). "Cytoplasm-to-vacuole targeting and autophagy employ the same machinery to deliver proteins to the yeast vacuole". Proceedings of the National Academy of Sciences of the United States of America. 93 (22): 12304–8. Bibcode:1996PNAS...9312304S. doi:10.1073/pnas.93.22.12304. PMC 37986. PMID 8901576.
- ↑ Klionsky DJ, Cregg JM, Dunn WA, Emr SD, Sakai Y, Sandoval IV, Sibirny A, Subramani S, Thumm M, Veenhuis M, Ohsumi Y (October 2003). "A unified nomenclature for yeast autophagy-related genes". Developmental Cell. 5 (4): 539–45. doi:10.1016/s1534-5807(03)00296-x. hdl:11370/221542fb-cff5-4604-a588-49ee7a7c84fb. PMID 14536056. S2CID 39590247.
- ↑ Van Noorden R, Ledford H (October 2016). "Medicine Nobel for research on how cells 'eat themselves'". Nature. 538 (7623): 18–19. Bibcode:2016Natur.538...18V. doi:10.1038/nature.2016.20721. PMID 27708326.
- 1 2 Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B (December 1999). "Induction of autophagy and inhibition of tumorigenesis by beclin 1". Nature. 402 (6762): 672–6. Bibcode:1999Natur.402..672L. doi:10.1038/45257. PMID 10604474. S2CID 4423132.
- ↑ "Autophagy in Stress, Development & Disease". Gordon Research Conference. 2003.
- ↑ "Autophagy in Health and Disease (Z3)". Keystone Symposia on Molecular and Cellular Biology. 2007. Archived from the original on 2018-11-16. Retrieved 2016-10-04.
- 1 2 Lee J, Giordano S, Zhang J (January 2012). "Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling". The Biochemical Journal. 441 (2): 523–40. doi:10.1042/BJ20111451. PMC 3258656. PMID 22187934.
- 1 2 3 4 Mizushima N, Ohsumi Y, Yoshimori T (December 2002). "Autophagosome formation in mammalian cells". Cell Structure and Function. 27 (6): 421–9. doi:10.1247/csf.27.421. PMID 12576635.
- 1 2 Youle RJ, Narendra DP (January 2011). "Mechanisms of mitophagy". Nature Reviews Molecular Cell Biology. 12 (1): 9–14. doi:10.1038/nrm3028. PMC 4780047. PMID 21179058.
- ↑ Ding WX, Yin XM (July 2012). "Mitophagy: mechanisms, pathophysiological roles, and analysis". Biological Chemistry. 393 (7): 547–64. doi:10.1515/hsz-2012-0119. PMC 3630798. PMID 22944659.
- 1 2 Liu K, Czaja MJ (January 2013). "Regulation of lipid stores and metabolism by lipophagy". Cell Death and Differentiation. 20 (1): 3–11. doi:10.1038/cdd.2012.63. PMC 3524634. PMID 22595754.
- ↑ Till A, Lakhani R, Burnett SF, Subramani S (2012). "Pexophagy: the selective degradation of peroxisomes". International Journal of Cell Biology. 2012: 512721. doi:10.1155/2012/512721. PMC 3320016. PMID 22536249.
- ↑ Lei L (March 2017). "Chlorophagy: Preventing sunburn". Nature Plants. 3 (3): 17026. doi:10.1038/nplants.2017.26. PMID 28248315. S2CID 30079770.
- ↑ An H, Harper JW (February 2018). "Systematic analysis of ribophagy in human cells reveals bystander flux during selective autophagy". Nature Cell Biology. 20 (2): 135–143. doi:10.1038/s41556-017-0007-x. PMC 5786475. PMID 29230017.
- 1 2 Levine B, Mizushima N, Virgin HW (January 2011). "Autophagy in immunity and inflammation". Nature. 469 (7330): 323–35. Bibcode:2011Natur.469..323L. doi:10.1038/nature09782. PMC 3131688. PMID 21248839.
- 1 2 3 Česen MH, Pegan K, Spes A, Turk B (July 2012). "Lysosomal pathways to cell death and their therapeutic applications". Experimental Cell Research. 318 (11): 1245–51. doi:10.1016/j.yexcr.2012.03.005. PMID 22465226.
- ↑ Avin-Wittenberg T, Honig A, Galili G (April 2012). "Variations on a theme: plant autophagy in comparison to yeast and mammals". Protoplasma. 249 (2): 285–99. doi:10.1007/s00709-011-0296-z. PMID 21660427. S2CID 17184033.
- 1 2 Homma KS (2011). "List of autophagy-related proteins and 3D structures". Autophagy Database. 290. Archived from the original on 2012-08-01. Retrieved 2012-10-08.
- ↑ Castro-Obregon S (2010). "The Discovery of Lysosomes and Autophagy". Nature Education. 3 (9): 49.
- ↑ Bandyopadhyay U, Kaushik S, Varticovski L, Cuervo AM (September 2008). "The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane". Molecular and Cellular Biology. 28 (18): 5747–63. doi:10.1128/MCB.02070-07. PMC 2546938. PMID 18644871.
- ↑ Ward C, Martinez-Lopez N, Otten EG, Carroll B, Maetzel D, Singh R, Sarkar S, Korolchuk VI (April 2016). "Autophagy, lipophagy and lysosomal lipid storage disorders". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 1861 (4): 269–84. doi:10.1016/j.bbalip.2016.01.006. hdl:1983/54269830-a38f-4546-93c2-424823701904. PMID 26778751.
- ↑ Elander PH, Minina EA, Bozhkov PV (March 2018). "Autophagy in turnover of lipid stores: trans-kingdom comparison". Journal of Experimental Botany. 69 (6): 1301–1311. doi:10.1093/jxb/erx433. PMID 29309625.
- ↑ van Zutphen T, Todde V, de Boer R, Kreim M, Hofbauer HF, Wolinski H, Veenhuis M, van der Klei IJ, Kohlwein SD (January 2014). "Lipid droplet autophagy in the yeast Saccharomyces cerevisiae". Molecular Biology of the Cell. 25 (2): 290–301. doi:10.1091/mbc.E13-08-0448. PMC 3890349. PMID 24258026.
- ↑ Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ (April 2009). "Autophagy regulates lipid metabolism". Nature. 458 (7242): 1131–5. Bibcode:2009Natur.458.1131S. doi:10.1038/nature07976. PMC 2676208. PMID 19339967.
- 1 2 Sudhakar P, Jacomin AC, Hautefort I, Samavedam S, Fatemian K, Ari E, et al. (September 2019). "Targeted interplay between bacterial pathogens and host autophagy". Autophagy. 15 (9): 1620–1633. doi:10.1080/15548627.2019.1590519. PMC 6693458. PMID 30909843.
 Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - 1 2 Klionsky DJ (September 2012). "Look people, "Atg" is an abbreviation for "autophagy-related." That's it". Autophagy. 8 (9): 1281–2. doi:10.4161/auto.21812. PMC 3442874. PMID 22889836.
- 1 2 Lamb CA, Yoshimori T, Tooze SA (December 2013). "The autophagosome: origins unknown, biogenesis complex". Nature Reviews. Molecular Cell Biology. 14 (12): 759–74. doi:10.1038/nrm3696. PMID 24201109. S2CID 24083190.
- ↑ Suzuki H, Osawa T, Fujioka Y, Noda NN (April 2017). "Structural biology of the core autophagy machinery". Current Opinion in Structural Biology. 43: 10–17. doi:10.1016/j.sbi.2016.09.010. PMID 27723509.
- ↑ Russell RC, Yuan HX, Guan KL (January 2014). "Autophagy regulation by nutrient signaling". Cell Research. 24 (1): 42–57. doi:10.1038/cr.2013.166. PMC 3879708. PMID 24343578.
- ↑ Chan EY (September 2012). "Regulation and function of uncoordinated-51 like kinase proteins". Antioxidants & Redox Signaling. 17 (5): 775–85. doi:10.1089/ars.2011.4396. PMID 22074133.
- ↑ Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J, Kim H, Neufeld TP, Dillin A, Guan KL (July 2013). "ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase". Nature Cell Biology. 15 (7): 741–50. doi:10.1038/ncb2757. PMC 3885611. PMID 23685627.
- ↑ Itakura E, Kishi C, Inoue K, Mizushima N (December 2008). "Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG". Molecular Biology of the Cell. 19 (12): 5360–72. doi:10.1091/mbc.E08-01-0080. PMC 2592660. PMID 18843052.
- ↑ Kang R, Zeh HJ, Lotze MT, Tang D (April 2011). "The Beclin 1 network regulates autophagy and apoptosis". Cell Death and Differentiation. 18 (4): 571–80. doi:10.1038/cdd.2010.191. PMC 3131912. PMID 21311563.
- ↑ Di Bartolomeo S, Corazzari M, Nazio F, Oliverio S, Lisi G, Antonioli M, Pagliarini V, Matteoni S, Fuoco C, Giunta L, D'Amelio M, Nardacci R, Romagnoli A, Piacentini M, Cecconi F, Fimia GM (October 2010). "The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy". The Journal of Cell Biology. 191 (1): 155–68. doi:10.1083/jcb.201002100. PMC 2953445. PMID 20921139.
- ↑ Hara T, Takamura A, Kishi C, Iemura S, Natsume T, Guan JL, Mizushima N (May 2008). "FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells". The Journal of Cell Biology. 181 (3): 497–510. doi:10.1083/jcb.200712064. PMC 2364687. PMID 18443221.
- ↑ T. Proikas-Cézanne, Z. Takacs, P. Donnes, and O. Kohlbacher, 'Wipi Proteins: Essential Ptdins3p Effectors at the Nascent Autophagosome', J Cell Sci, 128 (2015), 207-17
- 1 2 Kumar, Suresh; Javed, Ruheena; Mudd, Michal; Pallikkuth, Sandeep; Lidke, Keith A.; Jain, Ashish; Tangavelou, Karthikeyan; Gudmundsson, Sigurdur Runar; Ye, Chunyan; Rusten, Tor Erik; Anonsen, Jan Haug (November 2021). "Mammalian hybrid pre-autophagosomal structure HyPAS generates autophagosomes". Cell. 184 (24): 5950–5969.e22. doi:10.1016/j.cell.2021.10.017. PMC 8616855. PMID 34741801.
- ↑ Dooley HC, Razi M, Polson HE, Girardin SE, Wilson MI, Tooze SA (July 2014). "WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1". Molecular Cell. 55 (2): 238–52. doi:10.1016/j.molcel.2014.05.021. PMC 4104028. PMID 24954904.
- ↑ Hanada T, Noda NN, Satomi Y, Ichimura Y, Fujioka Y, Takao T, Inagaki F, Ohsumi Y (December 2007). "The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy". The Journal of Biological Chemistry. 282 (52): 37298–302. doi:10.1074/jbc.C700195200. PMID 17986448.
- ↑ Kabeya Y, Mizushima N, Yamamoto A, Oshitani-Okamoto S, Ohsumi Y, Yoshimori T (June 2004). "LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation". Journal of Cell Science. 117 (Pt 13): 2805–12. doi:10.1242/jcs.01131. PMID 15169837.
- ↑ Fujita N, Hayashi-Nishino M, Fukumoto H, Omori H, Yamamoto A, Noda T, Yoshimori T (November 2008). "An Atg4B mutant hampers the lipidation of LC3 paralogues and causes defects in autophagosome closure". Molecular Biology of the Cell. 19 (11): 4651–9. doi:10.1091/mbc.e08-03-0312. PMC 2575160. PMID 18768752.
- ↑ Park S, Choi SG, Yoo SM, Son JH, Jung YK (2014). "Choline dehydrogenase interacts with SQSTM1/p62 to recruit LC3 and stimulate mitophagy". Autophagy. 10 (11): 1906–20. doi:10.4161/auto.32177. PMC 4502719. PMID 25483962.
- ↑ Fader CM, Sánchez DG, Mestre MB, Colombo MI (December 2009). "TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1793 (12): 1901–16. doi:10.1016/j.bbamcr.2009.09.011. PMID 19781582.
- ↑ Furuta N, Fujita N, Noda T, Yoshimori T, Amano A (March 2010). "Combinational soluble N-ethylmaleimide-sensitive factor attachment protein receptor proteins VAMP8 and Vti1b mediate fusion of antimicrobial and canonical autophagosomes with lysosomes". Molecular Biology of the Cell. 21 (6): 1001–10. doi:10.1091/mbc.e09-08-0693. PMC 2836953. PMID 20089838.
- ↑ Kim YM, Jung CH, Seo M, Kim EK, Park JM, Bae SS, Kim DH (January 2015). "mTORC1 phosphorylates UVRAG to negatively regulate autophagosome and endosome maturation". Molecular Cell. 57 (2): 207–18. doi:10.1016/j.molcel.2014.11.013. PMC 4304967. PMID 25533187.
- ↑ Satoo K, Noda NN, Kumeta H, Fujioka Y, Mizushima N, Ohsumi Y, Inagaki F (May 2009). "The structure of Atg4B-LC3 complex reveals the mechanism of LC3 processing and delipidation during autophagy". The EMBO Journal. 28 (9): 1341–50. doi:10.1038/emboj.2009.80. PMC 2683054. PMID 19322194.
- ↑ Yang Z, Huang J, Geng J, Nair U, Klionsky DJ (December 2006). "Atg22 recycles amino acids to link the degradative and recycling functions of autophagy". Molecular Biology of the Cell. 17 (12): 5094–104. doi:10.1091/mbc.e06-06-0479. PMC 1679675. PMID 17021250.
- ↑ Yessenkyzy A, Saliev T, Zhanaliyeva M, Nurgozhin T (2020). "Polyphenols as Caloric-Restriction Mimetics and Autophagy Inducers in Aging Research". Nutrients. 12 (5): 1344. doi:10.3390/nu12051344. PMC 7285205. PMID 32397145.
- ↑ Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T (March 2008). "A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy". Proceedings of the National Academy of Sciences of the United States of America. 105 (9): 3374–89. Bibcode:2008PNAS..105.3374L. doi:10.1073/pnas.0712145105. PMC 2265142. PMID 18296641.
- ↑ Reggiori F, Klionsky DJ (February 2002). "Autophagy in the eukaryotic cell". Eukaryotic Cell. 1 (1): 11–21. doi:10.1128/EC.01.1.11-21.2002. PMC 118053. PMID 12455967.
- ↑ Klionsky DJ, Emr SD (December 2000). "Autophagy as a regulated pathway of cellular degradation". Science. 290 (5497): 1717–21. Bibcode:2000Sci...290.1717K. doi:10.1126/science.290.5497.1717. PMC 2732363. PMID 11099404.
- ↑ Levine B, Klionsky DJ (April 2004). "Development by self-digestion: molecular mechanisms and biological functions of autophagy". Developmental Cell. 6 (4): 463–77. doi:10.1016/S1534-5807(04)00099-1. PMID 15068787.
- ↑ Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, et al. (December 2004). "The role of autophagy during the early neonatal starvation period". Nature. 432 (7020): 1032–6. Bibcode:2004Natur.432.1032K. doi:10.1038/nature03029. PMID 15525940. S2CID 4424974.
- 1 2 Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y (March 2004). "In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker". Molecular Biology of the Cell. 15 (3): 1101–11. doi:10.1091/mbc.E03-09-0704. PMC 363084. PMID 14699058.
- 1 2 Tsukada M, Ohsumi Y (October 1993). "Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae". FEBS Letters. 333 (1–2): 169–74. doi:10.1016/0014-5793(93)80398-E. PMID 8224160. S2CID 46017791.
- ↑ Jackson WT, Giddings TH, Taylor MP, Mulinyawe S, Rabinovitch M, Kopito RR, Kirkegaard K (May 2005). "Subversion of cellular autophagosomal machinery by RNA viruses". PLOS Biology. 3 (5): e156. doi:10.1371/journal.pbio.0030156. PMC 1084330. PMID 15884975.

- ↑ Thurston TL, Wandel MP, von Muhlinen N, Foeglein A, Randow F (January 2012). "Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion". Nature. 482 (7385): 414–8. Bibcode:2012Natur.482..414T. doi:10.1038/nature10744. PMC 3343631. PMID 22246324.
- ↑ Cuervo AM, Bergamini E, Brunk UT, Dröge W, Ffrench M, Terman A (2005). "Autophagy and aging: the importance of maintaining "clean" cells". Autophagy. 1 (3): 131–40. doi:10.4161/auto.1.3.2017. PMID 16874025.
- 1 2 3 Tavassoly I (2015). Dynamics of Cell Fate Decision Mediated by the Interplay of Autophagy and Apoptosis in Cancer Cells. Springer Theses. Springer International Publishing. doi:10.1007/978-3-319-14962-2. ISBN 978-3-319-14962-2. S2CID 89307028.
- ↑ Tsujimoto Y, Shimizu S (November 2005). "Another way to die: autophagic programmed cell death". Cell Death and Differentiation. 12 (Suppl 2): 1528–34. doi:10.1038/sj.cdd.4401777. PMID 16247500.
- ↑ Schwartz LM, Smith SW, Jones ME, Osborne BA (February 1993). "Do all programmed cell deaths occur via apoptosis?". Proceedings of the National Academy of Sciences of the United States of America. 90 (3): 980–4. Bibcode:1993PNAS...90..980S. doi:10.1073/pnas.90.3.980. PMC 45794. PMID 8430112.
- ↑ Datan E, Shirazian A, Benjamin S, Matassov D, Tinari A, Malorni W, Lockshin RA, Garcia-Sastre A, Zakeri Z (March 2014). "mTOR/p70S6K signaling distinguishes routine, maintenance-level autophagy from autophagic cell death during influenza A infection". Virology. 452–453 (March 2014): 175–190. doi:10.1016/j.virol.2014.01.008. PMC 4005847. PMID 24606695.
- ↑ Sanchez AM, Bernardi H, Py G, Candau RB (October 2014). "Autophagy is essential to support skeletal muscle plasticity in response to endurance exercise". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 307 (8): R956-69. doi:10.1152/ajpregu.00187.2014. PMID 25121614.
- 1 2 Nair U, Klionsky DJ (December 2011). "Activation of autophagy is required for muscle homeostasis during physical exercise". Autophagy. 7 (12): 1405–6. doi:10.4161/auto.7.12.18315. PMC 3288013. PMID 22082869.
- 1 2 He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, et al. (January 2012). "Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis". Nature. 481 (7382): 511–5. Bibcode:2012Natur.481..511H. doi:10.1038/nature10758. PMC 3518436. PMID 22258505.
- 1 2 Grumati P, Coletto L, Schiavinato A, Castagnaro S, Bertaggia E, Sandri M, Bonaldo P (December 2011). "Physical exercise stimulates autophagy in normal skeletal muscles but is detrimental for collagen VI-deficient muscles". Autophagy. 7 (12): 1415–23. doi:10.4161/auto.7.12.17877. PMC 3288016. PMID 22024752.
- 1 2 Arndt V, Dick N, Tawo R, Dreiseidler M, Wenzel D, Hesse M, Fürst DO, Saftig P, Saint R, Fleischmann BK, Hoch M, Höhfeld J (January 2010). "Chaperone-assisted selective autophagy is essential for muscle maintenance". Current Biology. 20 (2): 143–8. doi:10.1016/j.cub.2009.11.022. PMID 20060297. S2CID 8885338.
- ↑ Ulbricht A, Eppler FJ, Tapia VE, van der Ven PF, Hampe N, Hersch N, Vakeel P, Stadel D, Haas A, Saftig P, Behrends C, Fürst DO, Volkmer R, Hoffmann B, Kolanus W, Höhfeld J (March 2013). "Cellular mechanotransduction relies on tension-induced and chaperone-assisted autophagy". Current Biology. 23 (5): 430–5. doi:10.1016/j.cub.2013.01.064. PMID 23434281.
- ↑ Caramés B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M (March 2010). "Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis". Arthritis and Rheumatism. 62 (3): 791–801. doi:10.1002/art.27305. PMC 2838960. PMID 20187128.
- ↑ Caramés B, Taniguchi N, Seino D, Blanco FJ, D'Lima D, Lotz M (April 2012). "Mechanical injury suppresses autophagy regulators and pharmacologic activation of autophagy results in chondroprotection". Arthritis and Rheumatism. 64 (4): 1182–92. doi:10.1002/art.33444. PMC 3288456. PMID 22034068.
- ↑ Caramés B, Olmer M, Kiosses WB, Lotz MK (June 2015). "The relationship of autophagy defects to cartilage damage during joint aging in a mouse model". Arthritis & Rheumatology. 67 (6): 1568–76. doi:10.1002/art.39073. PMC 4446178. PMID 25708836.
- 1 2 Furuya, N., Liang, X.H., and Levin, B. 2004. Autophagy and cancer. In Autophagy. D.J. Klionsky editor. Landes Bioscience. Georgetown, Texas, USA. 244-253.
- ↑ Vlahopoulos S, Critselis E, Voutsas IF, Perez SA, Moschovi M, Baxevanis CN, Chrousos GP (2014). "New use for old drugs? Prospective targets of chloroquines in cancer therapy". Current Drug Targets. 15 (9): 843–51. doi:10.2174/1389450115666140714121514. PMID 25023646.
- ↑ Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, et al. (December 2003). "Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene". The Journal of Clinical Investigation. 112 (12): 1809–20. doi:10.1172/JCI20039. PMC 297002. PMID 14638851.
- ↑ Duran A, Linares JF, Galvez AS, Wikenheiser K, Flores JM, Diaz-Meco MT, Moscat J (April 2008). "The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis". Cancer Cell. 13 (4): 343–54. doi:10.1016/j.ccr.2008.02.001. PMID 18394557.
- 1 2 3 Yang ZJ, Chee CE, Huang S, Sinicrope FA (September 2011). "The role of autophagy in cancer: therapeutic implications". Molecular Cancer Therapeutics. 10 (9): 1533–41. doi:10.1158/1535-7163.MCT-11-0047. PMC 3170456. PMID 21878654.
- 1 2 3 Tavassoly I, Parmar J, Shajahan-Haq AN, Clarke R, Baumann WT, Tyson JJ (April 2015). "Dynamic Modeling of the Interaction Between Autophagy and Apoptosis in Mammalian Cells". CPT: Pharmacometrics & Systems Pharmacology. 4 (4): 263–72. doi:10.1002/psp4.29. PMC 4429580. PMID 26225250.
- 1 2 3 Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, Domingo D, Yahalom J (January 2001). "A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles". Cancer Research. 61 (2): 439–44. PMID 11212227.
- ↑ Dökümcü K, Simonian M, Farahani RM (October 2018). "miR4673 improves fitness profile of neoplastic cells by induction of autophagy". Cell Death & Disease. 9 (11): 1068. doi:10.1038/s41419-018-1088-6. PMC 6195512. PMID 30341280.
- 1 2 3 Jin S, White E (2007). "Role of autophagy in cancer: management of metabolic stress". Autophagy. 3 (1): 28–31. doi:10.4161/auto.3269. PMC 2770734. PMID 16969128.
- ↑ Razaghi A, Heimann K, Schaeffer PM, Gibson SB (February 2018). "Negative regulators of cell death pathways in cancer: perspective on biomarkers and targeted therapies". Apoptosis. 23 (2): 93–112. doi:10.1007/s10495-018-1440-4. PMID 29322476. S2CID 3424489.
- ↑ Cadwell K (November 2016). "Crosstalk between autophagy and inflammatory signalling pathways: balancing defence and homeostasis". Nature Reviews. Immunology. 16 (11): 661–675. doi:10.1038/nri.2016.100. PMC 5343289. PMID 27694913.
- ↑ Medzhitov R (July 2008). "Origin and physiological roles of inflammation". Nature. 454 (7203): 428–35. Bibcode:2008Natur.454..428M. doi:10.1038/nature07201. PMID 18650913. S2CID 205214291.
- ↑ Tan P, Ye Y, Mao J, He L (2019). "Autophagy and Immune-Related Diseases". Autophagy Regulation of Innate Immunity. Advances in Experimental Medicine and Biology. Vol. 1209. pp. 167–179. doi:10.1007/978-981-15-0606-2_10. ISBN 978-981-15-0605-5. PMID 31728870. S2CID 208035547.
- ↑ Varisli L, Cen O, Vlahopoulos S (March 2020). "Dissecting pharmacological effects of chloroquine in cancer treatment: interference with inflammatory signaling pathways". Immunology. 159 (3): 257–278. doi:10.1111/imm.13160. PMC 7011648. PMID 31782148.
- ↑ Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, González-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW (May 2004). "Hereditary early-onset Parkinson's disease caused by mutations in PINK1". Science. 304 (5674): 1158–60. Bibcode:2004Sci...304.1158V. doi:10.1126/science.1096284. PMID 15087508. S2CID 33630092.
- ↑ Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N (April 1998). "Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism". Nature. 392 (6676): 605–8. Bibcode:1998Natur.392..605K. doi:10.1038/33416. PMID 9560156. S2CID 4432261.
- ↑ Esteves AR, Arduíno DM, Silva DF, Oliveira CR, Cardoso SM (January 2011). "Mitochondrial Dysfunction: The Road to Alpha-Synuclein Oligomerization in PD". Parkinson's Disease. 2011: 693761. doi:10.4061/2011/693761. PMC 3026982. PMID 21318163.
- ↑ Moosavi MA, Haghi A, Rahmati M, Taniguchi H, Mocan A, Echeverría J, Gupta VK, Tzvetkov NT, Atanasov AG (2018). "Phytochemicals as potent modulators of autophagy for cancer therapy". Cancer Lett. 424: 46–69. doi:10.1016/j.canlet.2018.02.030. PMID 29474859. S2CID 4458797.
Further reading
- Liu Y, Bassham DC (2012). "Autophagy: pathways for self-eating in plant cells". Annual Review of Plant Biology. 63: 215–37. doi:10.1146/annurev-arplant-042811-105441. PMID 22242963.
- Starokadomskyy P, Dmytruk KV (July 2013). "A bird's-eye view of autophagy". Autophagy. 9 (7): 1121–6. doi:10.4161/auto.24544. PMC 3722328. PMID 23615436.
- Tavassoly I (February 2015). Dynamics of Cell Fate Decision Mediated by the Interplay of Autophagy and Apoptosis in Cancer Cells: Mathematical Modeling and Experimental Observations. Springer Theses. Springer. doi:10.1007/978-3-319-14962-2. ISBN 978-3-319-14961-5. S2CID 89307028.
- "Function and mechanism of autophagy and its key points". Biomentors.net.
External links
- Autophagy, a journal produced by Landes Bioscience and edited by DJ Klionsky
- LongevityMeme entry describing PubMed article on the effects of autophagy and lifespan
- Autophagolysosome on Drugs.com
- HADb, a Human Autophagy dedicated Database
- Autophagy DB, an autophagy database that covers all eukaryotes
- Self-Destructive Behavior in Cells May Hold Key to a Longer Life
- Exercise as Housecleaning for the Body
- The AIM center