 | |
| Names | |
|---|---|
| Other names
Aizen Cathilon Red GTLH, Astrazon Red GTL | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.034.482 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
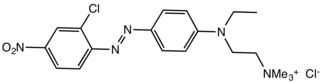
| C19H25Cl2N5O2 | |
| Molar mass | 426.34 g·mol−1 |
| Appearance | dark red solid |
| Melting point | 45.5–46.5 °C (113.9–115.7 °F; 318.6–319.6 K) |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H302, H318, H412 | |
| P264, P270, P273, P280, P301+P312, P305+P351+P338, P310, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Basic Red 18 is a cationic azo dye used for coloring textiles. The chromophore is the cation, which contains many functional groups, but most prominently the quaternary ammonium center.
It is produced by azo coupling of 2-chloro-4-nitrophenyldiazonium cation with the quaternary ammonium salt derived from N-ethyl-N-(2-chloroethyl)aniline and trimethylamine.[1]
Like many dyes, methods for the removal of Basic Red 18 from waste streams has received much attention.[2]
References
- ↑ Klaus Hunger; Peter Mischke; Wolfgang Rieper; et al. (2005). "Azo Dyes". Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_245. ISBN 3527306730..
- ↑ B.Noroozi; G. A.Sorial; H.Bahrami; M.Arami (2008). "Adsorption of binary mixtures of cationic dyes". Dyes and Pigments. 76 (3): 784–791. doi:10.1016/j.dyepig.2007.02.003.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.