 | |
| Names | |
|---|---|
| Systematic IUPAC name
Hexa-μ-acetato(O,O')-μ4-oxo-tetraberyllium(II) | |
| Other names
Beryllium oxyacetate Beryllium oxide acetate Basic beryllium acetate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.038.881 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C 12H 18Be 4O 13 | |
| Molar mass | 406.3122 g/mol |
| Appearance | colorless |
| Melting point | 285 °C (545 °F; 558 K) |
| Boiling point | 330 °C (626 °F; 603 K) |
| Solubility in chloroform | soluble |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
highly toxic |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 0.002 mg/m3 C 0.005 mg/m3 (30 minutes), with a maximum peak of 0.025 mg/m3 (as Be)[1] |
REL (Recommended) |
Ca C 0.0005 mg/m3 (as Be)[1] |
IDLH (Immediate danger) |
Ca [4 mg/m3 (as Be)][1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
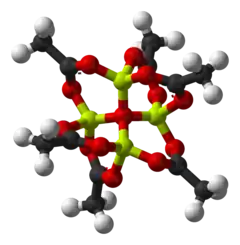
Basic beryllium acetate is the chemical compound with the formula Be4O(O2CCH3)6. This compound adopts a distinctive structure, but it has no applications and has been only lightly studied. It is a colourless solid that is soluble in organic solvents.
Preparation
It can be prepared by treating basic beryllium carbonate with hot acetic acid.
- 2 Be2CO3(OH)2 + 6 AcOH → Be4O(AcO)6 + 5 H2O + 2 CO2
Basic beryllium acetate is insoluble in water but soluble in chloroform, consistent with it being nonpolar. It melts and sublimes in a vacuum without decomposition.[2]
Structure
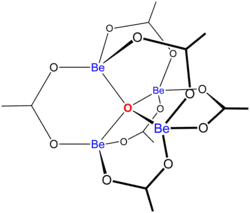
"Basic acetates" consist of an ensemble of metal centres bound to a central oxide ion, and a collection of acetate ligands. Basic beryllium acetate has a tetrahedral Be4O6+ core with acetates (CH3CO2−) spanning each of the pairs of Be2+ centres.[3][4] It consists of interlocking six-membered Be2O3C rings. The structure is relevant to its considerable stability (the compound is distillable at 330 °C).
Uses
The solubility of the salt in organic solvents (chloroform) is useful to extract and purify beryllium rich fractions for many purposes. Basic beryllium acetate single crystals can easily be grown and are helpful to align x-ray diffractometers and also as a reference in protein crystallography.
See also
References
- 1 2 3 NIOSH Pocket Guide to Chemical Hazards. "#0054". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Moeller, T. (1950). "Basic Beryllium Derivatives of Organic Acids". In Audrieth, L. F. (ed.). Inorganic Syntheses, Volume 3. Vol. 3. John Wiley & Sons. pp. 4–9. doi:10.1002/9780470132340.ch2. ISBN 978-0-470-13234-0.
- ↑ Bragg, W. H. (1923). "Crystal Structure of Basic Beryllium Acetate". Nature. 111 (2790): 532. Bibcode:1923Natur.111..532B. doi:10.1038/111532a0.
- ↑ Pauling, L.; Sherman, J. (1934). "The Structure of the Carboxyl Group. II. The Crystal Structure of Basic Beryllium Acetate" (PDF). Proceedings of the National Academy of Sciences. 20 (6): 340–5. Bibcode:1934PNAS...20..340P. doi:10.1073/pnas.20.6.340. PMC 1076415. PMID 16587899.