 | |
 | |
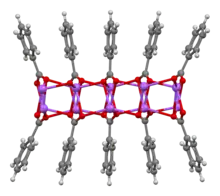 Ball-and-stick model of part of the crystal structure | |
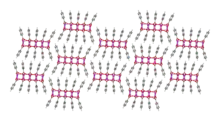 Ball-and-stick model of packing in the crystal structure | |
| Names | |
|---|---|
| Preferred IUPAC name
Sodium benzoate | |
| Other names
E211, benzoate of soda | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.760 |
| E number | E211 (preservatives) |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H5NaO2 | |
| Molar mass | 144.105 g·mol−1 |
| Appearance | white or colourless crystalline powder |
| Odor | odorless |
| Density | 1.497 g/cm3 |
| Melting point | 410 °C (770 °F; 683 K) |
| 62.65 g/100 mL (0 °C) 62.84 g/100 mL (15 °C) 62.87 g/100 mL (30 °C) 74.2 g/100 mL (100 °C)[1] | |
| Solubility | soluble in liquid ammonia, pyridine[1] |
| Solubility in methanol | 8.22 g/100 g (15 °C) 7.55 g/100 g (66.2 °C)[1] |
| Solubility in ethanol | 2.3 g/100 g (25 °C) 8.3 g/100 g (78 °C)[1] |
| Solubility in 1,4-Dioxane | 0.818 mg/kg (25 °C)[1] |
| Pharmacology | |
| A16AX11 (WHO) | |
| Hazards | |
| GHS labelling: | |
 [2] [2] | |
| Warning | |
| H319[2] | |
| P305+P351+P338[2] | |
| NFPA 704 (fire diamond) | |
| Flash point | 100 °C (212 °F; 373 K) |
| 500 °C (932 °F; 773 K) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
4100 mg/kg (oral, rat) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
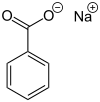
Sodium benzoate also known as benzoate of soda is the sodium salt of benzoic acid, widely used as a food preservative (with an E number of E211) and a pickling agent. It appears as a white crystalline chemical with the formula C6H5COONa.
Production
Sodium benzoate is commonly produced by the neutralization of sodium hydroxide (NaOH) with benzoic acid (C6H5COOH),[3] which is itself produced commercially by partial oxidation of toluene with oxygen.
Reactions
Sodium benzoate can be decarboxylated with strong base and heat, yielding benzene:[4]
Natural occurrence
Many foods are natural sources of benzoic acid, its salts, and its esters.[5] Fruits and vegetables can be rich sources, particularly berries such as cranberry and bilberry. Other sources include seafood, such as prawns, and dairy products.
Uses
As a preservative
Sodium benzoate can act as a food preservative. It is most widely used in acidic foods such as salad dressings (for example acetic acid in vinegar), carbonated drinks (carbonic acid), jams and fruit juices (citric acid), pickles (acetic acid), condiments, and frozen yogurt toppings. It is also used as a preservative in medicines and cosmetics.[6][7] Under these conditions it is converted into benzoic acid (E210), which is bacteriostatic and fungistatic. Benzoic acid is generally not used directly due to its poor water solubility. Concentration as a food preservative is limited by the FDA in the U.S. to 0.1% by weight.[8] Sodium benzoate is also allowed as an animal food additive at up to 0.1%, per the Association of American Feed Control Officials.[9] Sodium benzoate has been replaced by potassium sorbate in the majority of soft drinks in the United Kingdom.[10]
In the 19th century, sodium benzoate as a food ingredient was investigated by Harvey W. Wiley with his 'Poison Squad' as part of the US Department of Agriculture. This led to the 1906 Pure Food and Drug Act, a key event in the early history of food regulation in the United States.
In pharmaceuticals
Sodium benzoate is used as a treatment for urea cycle disorders due to its ability to bind amino acids.[11][12] This leads to excretion of these amino acids and a decrease in ammonia levels. Recent research shows that sodium benzoate may be beneficial as an add-on therapy (1 gram/day) in schizophrenia.[13][14][15] Total Positive and Negative Syndrome Scale scores dropped by 21% compared to placebo.
Sodium benzoate, along with phenylbutyrate, is used to treat hyperammonemia.[16][17]
Sodium benzoate, along with caffeine, is used to treat postdural puncture headache, respiratory depression associated with overdosage of narcotics,[18][19] and with ergotamine to treat vascular headache.[20]
Other uses
Sodium benzoate is also used in fireworks as a fuel in whistle mix, a powder that emits a whistling noise when compressed into a tube and ignited.
Mechanism of food preservation
The mechanism starts with the absorption of benzoic acid into the cell. If the intracellular pH falls to 5 or lower, the anaerobic fermentation of glucose through phosphofructokinase decreases sharply,[21] which inhibits the growth and survival of microorganisms that cause food spoilage.
Health and safety

In the United States, sodium benzoate is designated as generally recognized as safe (GRAS) by the Food and Drug Administration.[22] The International Programme on Chemical Safety found no adverse effects in humans at doses of 647–825 mg/kg of body weight per day.[23][24]
Cats have a significantly lower tolerance against benzoic acid and its salts than rats and mice.[25]
The human body rapidly clears sodium benzoate by combining it with glycine to form hippuric acid which is then excreted.[24] The metabolic pathway for this begins with the conversion of benzoate by butyrate-CoA ligase into an intermediate product, benzoyl-CoA,[26] which is then metabolized by glycine N-acyltransferase into hippuric acid.[27]
Association with benzene in soft drinks & pepper sauces
In combination with ascorbic acid (vitamin C, E300), sodium benzoate and potassium benzoate may form benzene. In 2006, the Food and Drug Administration tested 100 beverages available in the United States that contained both ascorbic acid and benzoate. Four had benzene levels that were above the 5 ppb Maximum Contaminant Level set by the Environmental Protection Agency for drinking water.[28] Most of the beverages that tested above the limit have been reformulated and subsequently tested below the safety limit.[28] Heat, light and shelf life can increase the rate at which benzene is formed. Hot peppers naturally contain vitamin C ("nearly as much as in one orange"[29]) so the observation about beverages applies to pepper sauces containing sodium benzoate, like Texas Pete.
ADHD and hyperactivity
Research published, including in 2007 for the UK's Food Standards Agency (FSA) suggests that certain artificial colors, when paired with sodium benzoate, may be linked to hyperactive behavior and other ADHD symptoms. The results were inconsistent regarding sodium benzoate, so the FSA recommended further study.[30][31][32] The Food Standards Agency concluded that the observed increases in hyperactive behavior, if real, were more likely to be linked to the artificial colors than to sodium benzoate.[32] The report's author, Jim Stevenson from Southampton University, said: "The results suggest that consumption of certain mixtures of artificial food colours and sodium benzoate preservative are associated with increases in hyperactive behaviour in children. . . . Many other influences are at work but this at least is one a child can avoid."[32]
Compendial status
See also
References
- 1 2 3 4 5 "sodium benzoate". chemister.ru.
- 1 2 3 Sigma-Aldrich Co., Sodium benzoate. Retrieved on 2014-05-23.
- ↑ "International Programme on Chemical Safety". Inchem.org. Retrieved 9 February 2022.
- ↑ Wertheim, Edgar (1942). Introductory Organic Chemistry with Certain Chapters of Biochemistry. The Blakistan Company. p. 236.
- ↑ del Olmo, Ana; Calzada, Javier; Nuñez, Manuel (20 November 2015). "Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy". Critical Reviews in Food Science and Nutrition. 57 (14): 3084–3103. doi:10.1080/10408398.2015.1087964. PMID 26587821. S2CID 205692543.
- ↑ "Sodium benzoate". PubChem. National Library of Medicine
- ↑ "Robitussin (Guaifenesin)". Rxmed.com. Retrieved 14 January 2013.
- ↑ "Code of Federal Regulations Title 21". www.accessdata.fda.gov.
- ↑ AAFCO (2004). "Official Publication": 262.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Saltmarsh, Mike (15 March 2015). "Recent trends in the use of food additives in the United Kingdom". Journal of the Science of Food and Agriculture. 95 (4): 649–652. Bibcode:2015JSFA...95..649S. doi:10.1002/jsfa.6715. ISSN 1097-0010. PMID 24789520.
... the preservative used in the study, sodium benzoate, has been replaced by potassium sorbate in the majority of soft drinks.
- ↑ Häberle, J; Boddaert, N; Burlina, A; Chakrapani, A; Dixon, M; Huemer, M; Karall, D; Martinelli, D; Crespo, PS; Santer, R; Servais, A; Valayannopoulos, V; Lindner, M; Rubio, V; Dionisi-Vici, C (2012). "Suggested guidelines for the diagnosis and management of urea cycle disorders". Orphanet Journal of Rare Diseases. 7: 32. doi:10.1186/1750-1172-7-32. PMC 3488504. PMID 22642880.
- ↑ Wilcken, B (2004). "Problems in the management of urea cycle disorders". Molecular Genetics and Metabolism. 81 (Suppl 1): S86–91. doi:10.1016/j.ymgme.2003.10.016. PMID 15050980.
- ↑ Add-on Treatment of Benzoate for Schizophrenia A Randomized, Double-blind, Placebo-Controlled Trial of d-Amino Acid Oxidase Inhibitor December 2013
- ↑ "Digest of Neurology and Psychiatry". Institute of Living. 16 April 2018 – via Google Books.
- ↑ Mental Health Research Institute Staff Publications, University of Michigan. Mental Health Research Institute
- ↑ "Cinnamon May Help Halt Parkinson's Disease Progression - News Releases - Rush University Medical Center".
- ↑ PHENYLBUTYRATE, SODIUM BENZOATE. National Institute of Diabetes and Digestive and Kidney Diseases. 2012.
- ↑ Yücel, A; Ozyalçin, S; Talu, GK; Yücel, EC; Erdine, S (1999). "Intravenous administration of caffeine sodium benzoate for postdural puncture headache". Reg Anesth Pain Med. 24 (1): 51–4. doi:10.1097/00115550-199924010-00010. PMID 9952095.
- ↑ mayoclinic.org, Caffeine And Sodium Benzoate (Injection Route)
- ↑ ebi.ac.uk, CHEBI:32140 - sodium caffeine benzoate
- ↑ Krebs H. A.; Wiggins D.; Stubbs M.; Sols A.; Bedoya F. (September 1983). "Studies on the mechanism of the antifungal action of benzoate". Biochemical Journal. 218 (3): 657–663. doi:10.1042/bj2140657. PMC 1152300. PMID 6226283.
- ↑ "CFR - Code of Federal Regulations Title 21". www.accessdata.fda.gov.
- ↑ "Concise International Chemical Assessment Document 26: Benzoic acid and sodium benzoate". Inchem.org. Retrieved 14 January 2013.
- 1 2 Cosmetic Ingredient Review Expert Panel Bindu Nair (2001). "Final Report on the Safety Assessment of Benzyl Alcohol, Benzoic Acid, and Sodium Benzoate". Int J Tox. 20 (Suppl 3): 23–50. doi:10.1080/10915810152630729. PMID 11766131. S2CID 13639993.
- ↑ Bedford PG, Clarke EG (January 1972). "Experimental benzoic acid poisoning in the cat". Vet. Rec. 90 (3): 53–8. doi:10.1136/vr.90.3.53. PMID 4672555. S2CID 2553612.
- ↑ "butyrate-CoA ligase". BRENDA. Technische Universität Braunschweig. Retrieved 7 May 2014. Substrate/Product
- ↑ "glycine N-acyltransferase". BRENDA. Technische Universität Braunschweig. Retrieved 7 May 2014. Substrate/Product
- 1 2 "Data on Benzene in Soft Drinks and Other Beverages". United States Food and Drug Administration. 16 May 2007. Archived from the original on 12 January 2017. Retrieved 7 November 2013.
- ↑ "Is hot sauce good for your health?". 4 November 2016.
- ↑ Food Standards Agency issues revised advice on certain artificial colours Archived 6 December 2011 at the Wayback Machine 6 September 2007
- ↑ Food Colorings and Hyperactivity Archived 20 May 2023 at the Wayback Machine "Myomancy" 7 September 2007
- 1 2 3 Agency revises advice on certain artificial colours Archived 12 April 2012 at the Wayback Machine, Food Standards Agency, 11 September 2007
- 1 2 3 Sigma Aldrich. "Sodium benzoate". Retrieved 17 July 2009.
- ↑ Therapeutic Goods Administration. "Chemical Substances" (PDF). Archived from the original (PDF) on 15 June 2009. Retrieved 17 July 2009.
- ↑ British Pharmacopoeia Commission Secretariat. "Index (BP)" (PDF). Archived from the original (PDF) on 11 April 2009. Retrieved 2 March 2010.
- ↑ "Japanese Pharmacopoeia 15th Edition". Archived from the original on 28 March 2010. Retrieved 2 March 2010.
- ↑ The United States Pharmacopeial Convention. "Revisions to USP 29–NF 24". Archived from the original on 4 April 2009. Retrieved 17 July 2009.
External links
- International Programme on Chemical Safety - Benzoic Acid and Sodium Benzoate report
- Kubota K, Ishizaki T (1991). "Dose-dependent pharmacokinetics of benzoic acid following oral administration of sodium benzoate to humans". Eur. J. Clin. Pharmacol. 41 (4): 363–8. doi:10.1007/BF00314969. PMID 1804654. S2CID 8196430.
Although the maximum rate of biotransformation of benzoic acid to hippuric acid varied between 17.2 and 28.8 mg.kg-1.h-1 among the six individuals, the mean value (23.0 mg.kg-1.h-1) was fairly close to that provided by daily maximum dose (0.5 g.kg-1.day-1) recommended in the treatment of hyperammonaemia in patients with inborn errors of ureagenesis
- Safety data for sodium benzoate Archived 11 December 2011 at the Wayback Machine
- The Ketchup Conundrum
