 | |
| Names | |
|---|---|
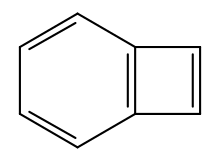
| Preferred IUPAC name
Bicyclo[4.2.0]octa-1,3,5,7-tetraene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H6 | |
| Molar mass | 102.136 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Benzocyclobutadiene is the simplest polycyclic hydrocarbon, being composed of an aromatic benzene ring fused to an anti-aromatic cyclobutadiene ring. It has chemical formula C8H6. Though the benzene ring is stabilized by aromaticity, the cyclobutadiene portion has a destabilizing effect. This results into it being a non-aromatic compound - neither behaving as aromatic nor an antiaromatic one.[1] For this reason, benzocyclobutadiene will readily dimerize or polymerize and it reacts as a dienophile in Diels-Alder reactions.[2]
Benzocyclobutadiene is used in the production of the pharmaceutical drug naflocort.
See also
References
- ↑ Observation of benzocyclobutadiene by flow nuclear magnetic resonance https://doi.org/10.1021/ja00168a059
- ↑ Carey, Francis A.; Sundberg, Richard J. (1984). Advanced Organic Chemistry Part A Structure and Mechanisms (2nd ed.). New York, NY: Plenum Press. ISBN 0-306-41198-9.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.