 | |
| Names | |
|---|---|
| IUPAC name
3β-Hydroxylup-20(29)-en-28-oic acid | |
| Systematic IUPAC name
(1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-Hydroxy-5a,5b,8,8,11a-pentamethyl-1-(prop-1-en-1-yl)icosahydro-3aH-cyclopenta[a]chrysene-3a-carboxylic acid | |
| Other names
Betulic acid Mairin | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.773 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C30H48O3 | |
| Molar mass | 456.711 g·mol−1 |
| Melting point | 316 to 318 °C (601 to 604 °F; 589 to 591 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Betulinic acid is a naturally occurring pentacyclic triterpenoid which has antiretroviral, antimalarial, and anti-inflammatory properties, as well as a more recently discovered potential as an anticancer agent, by inhibition of topoisomerase.[1] It is found in the bark of several species of plants, principally the white birch (Betula pubescens)[2] from which it gets its name, but also the ber tree (Ziziphus mauritiana), selfheal (Prunella vulgaris), the tropical carnivorous plants Triphyophyllum peltatum and Ancistrocladus heyneanus, Diospyros leucomelas, a member of the persimmon family, Tetracera boiviniana, the jambul (Syzygium formosanum),[3] flowering quince (Pseudocydonia sinensis, former Chaenomeles sinensis KOEHNE),[4] rosemary,[5] and Pulsatilla chinensis.[6]
Antitumor activity
In 1995, betulinic acid was reported as a selective inhibitor of human melanoma.[7] Then it was demonstrated to induce apoptosis in human neuroblastoma in vitro and in vivo in model systems.[8] At one time, it was undergoing drug development with assistance from the Rapid Access to Intervention Development program of the National Cancer Institute.[2] Also, betulinic acid was found active in vitro against neuroectodermal (neuroblastoma, medulloblastoma, Ewing's sarcoma[9]) and malignant brain tumors,[3][10] ovarian carcinoma,[3] in human leukemia HL-60 cells,[6] and malignant head and neck squamous cell carcinoma SCC25 and SCC9 cell lines.[11] In contrast, epithelial tumors, such as breast, colon, small cell lung and renal cell carcinomas, as well as T-cell leukemia cells, were completely unresponsive to treatment with betulinic acid.[9]
The effects of betulinic acid as an anticancer agent in breast cancer is found to be cannabinoid receptor dependent. Betulinic acid behaves as a CB1 antagonist and CB2 agonist.[12]
Mode of action
Regarding the mode of action of betulinic acid, little is known about its antiproliferative and apoptosis-inducing mechanisms. In neuroectodermal tumor cells, betulinic acid–induced apoptosis is accompanied by caspase activation, mitochondrial membrane alterations and DNA fragmentation.[9][11] Caspases are produced as inactive proenzymes, which are proteolytically processed to their active forms. These proteases can cooperate in proteolytic cascades, in which caspases activate themselves and each other. The initiation of the caspases cascade may lead to the activation of endonucleases such as caspase-activated DNAase (CAD). After activation, CAD contributes to DNA degradation.[11] Betulinic acid induces apoptosis by direct effects on mitochondria, leading to cytochrome-C release, which in turn regulates the "downstream" caspase activation.[11] Betulinic acid bypasses resistance to CD95 and doxorubicin-mediated apoptosis, due to different molecular mechanism of betulinic acid-induced apoptosis.
The role of p53 in betulinic acid-induced apoptosis is controversial. Fulda suggested a p53-independent mechanism of the apoptosis, based on no accumulation of wild-type p53 detected upon treatment with the betulinic acid, whereas wild-type p53 protein strongly increased after treatment with doxorubicin.[9] The suggestion is supported by study of Raisova.[13] Alternatively, Rieber suggested betulinic acid exerts its inhibitory effect on human metastatic melanoma partly by increasing p53.[14]
The study also demonstrated preferential apoptotic effect of betulinic acid on C8161 metastatic melanoma cells, with greater DNA fragmentation and growth arrest and earlier loss of viability than their nonmetastatic C8161/neo 6.3 counterpart.[14] Comparing betulinic acid with other treatment modes, Zuco demonstrated it was less than 10% as potent as doxorubicin and showed an in vitro antiproliferative activity against melanoma and nonmelanoma cell lines, including those resistant to doxorubicin. On the human normal dermatoblast cell line, betulinic acid was one-half to one-fifth as toxic as doxorubicin.[3] The ability of betulinic acid to induce two different effects (cytotoxic and cytostatic) on two clones derived from the same human melanoma metastasis suggests the development of clones resistant to this agent will be more unlikely, than that to conventional cytotoxic drugs. Moreover, in spite of the lower potency compared with doxorubicin, betulinic acid seems to be selective for tumor cells with minimal toxicity against normal cells.[3] The effect of betulinic acid on melanoma cell lines is stronger than its growth-inhibitory effect on primary melanocytes.[15] A study of a combination of betulinic acid with γ-irradiation showed clearly additive effects, and indicated they differ in their modes of action.[15]
C-3 esterification of betulinic acid led to the discovery of bevirimat, an HIV-1 maturation inhibitor patented by Rhone-Poulenc (now Sanofi-Aventis). The clinical development, however, was stopped due to poor pharmacodynamic properties.[16]
Use in cosmetics
There has been great emphasis on the use of betulinic acid as an antioxidative additive. Creams containing betulinic acid have been proven to help against highly reactive radicals that might cause skin DNA damage. Furthermore, betulinic acid was able to counteract the effects of ionizing radiation like UV. This makes betulinic acid a great additive for sunscreems and sunblocks and also creams for anti-aging purposes.[17]
Biosynthesis
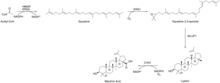
Saccharomyces cerevisiae has been engineered to produce betulinic acid from the mevalonate pathway, with squalene 2,3-epoxide as an intermediate. Acetyl-CoA is converted to squalene through use of the 3-hydroxyl-3-methylglutaryl-CoA reductase (HMGR) and the bifunctional farnesyl-diphosphate farnesyltransferase and squalene synthase (ERG9) and oxidation of NADPH to NADP+. This is then further oxygenated by the squalene monooxygenase (ERG1) to squalene 2,3-epoxide. This is cyclized to lupeol by the Arabidopsis thaliana lupeol synthase (AtLUP1). Finally, lupeol is converted to betulinic acid through the Catharanthus roseus P450 monooxygenase (CrAO) with the oxidation of NADPH to NADP+.[18]
Anticancer derivatives
A major inconvenience for the future clinical development of betulinic acid and analogues resides in their poor solubility in aqueous media such as blood serum and polar solvents used for bioassays. To circumvent this problem of hydrosolubility and to enhance pharmacological properties, many derivatives were synthesized and evaluated for cytotoxic activity. One study showed C-20 modifications involve the loss of cytotoxicity. Another study demonstrated the importance of the presence of the -COOH group, since compounds substituted at this position, such as lupeol and methyl betulinate, were less active on human melanoma than betulinic acid. Moreover, some C-28 amino acids and C-3 phthalates derivatives exhibited higher cytotoxic activity against cancer cell lines with improved selective toxicity and water solubility. Chatterjee et al. obtained the 28-O-β-D-glucopyranoside of betulinic acid by microbial transformation with Cunninghamella species, while Baglin et al. obtained it by organic synthesis. This glucoside did not exhibit any significant in vitro activity on human melanoma (MEL-2) and human colorectal adenocarcinoma (HT-29) cell lines, which confirms the importance of the carboxylic acid function to preserve the cytotoxicity. Recently, Gauthier et al. synthesized a series of 3-O-glycosides of betulinic acid which exhibited a strongly potent in vitro anticancer activity against human cancer cell lines.[19]
See also
References
- ↑ Chowdhury AR, Mandal S, Mittra B, Sharma S, Mukhopadhyay S, Majumder HK (July 2002). "Betulinic acid, a potent inhibitor of eukaryotic topoisomerase I: identification of the inhibitory step, the major functional group responsible and development of more potent derivatives". Medical Science Monitor. 8 (7): BR254–65. PMID 12118187.
- 1 2 Tan Y, Yu R, Pezzuto JM (July 2003). "Betulinic acid-induced programmed cell death in human melanoma cells involves mitogen-activated protein kinase activation". Clinical Cancer Research. 9 (7): 2866–75. PMID 12855667.
- 1 2 3 4 5 Zuco V, Supino R, Righetti SC, Cleris L, Marchesi E, Gambacorti-Passerini C, Formelli F (January 2002). "Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells". Cancer Letters. 175 (1): 17–25. doi:10.1016/S0304-3835(01)00718-2. PMID 11734332.
- ↑ Gao H, Wu L, Kuroyanagi M, Harada K, Kawahara N, Nakane T, Umehara K, Hirasawa A, Nakamura Y (November 2003). "Antitumor-promoting constituents from Chaenomeles sinensis KOEHNE and their activities in JB6 mouse epidermal cells". Chemical & Pharmaceutical Bulletin. 51 (11): 1318–21. doi:10.1248/cpb.51.1318. PMID 14600382. (Chaenomeles sinensis KOEHNE is now named Pseudocydonia sinensis)
- ↑ Abe F, Yamauchi T, Nagao T, Kinjo J, Okabe H, Higo H, Akahane H (November 2002). "Ursolic acid as a trypanocidal constituent in rosemary". Biological & Pharmaceutical Bulletin. 25 (11): 1485–7. doi:10.1248/bpb.25.1485. PMID 12419966.
- 1 2 Ji ZN, Ye WC, Liu GG, Hsiao WL (November 2002). "23-Hydroxybetulinic acid-mediated apoptosis is accompanied by decreases in bcl-2 expression and telomerase activity in HL-60 Cells". Life Sciences. 72 (1): 1–9. doi:10.1016/S0024-3205(02)02176-8. PMID 12409140.
- ↑ Pisha E, Chai H, Lee IS, Chagwedera TE, Farnsworth NR, Cordell GA, Beecher CW, Fong HH, Kinghorn AD, Brown DM (October 1995). "Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis". Nature Medicine. 1 (10): 1046–51. doi:10.1038/nm1095-1046. PMID 7489361. S2CID 24752850.
- ↑ Schmidt ML, Kuzmanoff KL, Ling-Indeck L, Pezzuto JM (October 1997). "Betulinic acid induces apoptosis in human neuroblastoma cell lines". European Journal of Cancer. 33 (12): 2007–10. doi:10.1016/S0959-8049(97)00294-3. PMID 9516843.
- 1 2 3 4 Fulda S, Friesen C, Los M, Scaffidi C, Mier W, Benedict M, Nuñez G, Krammer PH, Peter ME, Debatin KM (November 1997). "Betulinic acid triggers CD95 (APO-1/Fas)- and p53-independent apoptosis via activation of caspases in neuroectodermal tumors". Cancer Research. 57 (21): 4956–64. PMID 9354463.
- ↑ Wick W, Grimmel C, Wagenknecht B, Dichgans J, Weller M (June 1999). "Betulinic acid-induced apoptosis in glioma cells: A sequential requirement for new protein synthesis, formation of reactive oxygen species, and caspase processing". The Journal of Pharmacology and Experimental Therapeutics. 289 (3): 1306–12. PMID 10336521.
- 1 2 3 4 Thurnher D, Turhani D, Pelzmann M, Wannemacher B, Knerer B, Formanek M, Wacheck V, Selzer E (September 2003). "Betulinic acid: a new cytotoxic compound against malignant head and neck cancer cells". Head & Neck. 25 (9): 732–40. doi:10.1002/hed.10231. PMID 12953308. S2CID 24271002.
- ↑ Liu X, Jutooru I, Lei P, Kim K, Lee SO, Brents LK, Prather PL, Safe S (July 2012). "Betulinic acid targets YY1 and ErbB2 through cannabinoid receptor-dependent disruption of microRNA-27a:ZBTB10 in breast cancer". Molecular Cancer Therapeutics. 11 (7): 1421–31. doi:10.1158/1535-7163.MCT-12-0026. PMC 4924623. PMID 22553354.
- ↑ Raisova M, Hossini AM, Eberle J, Riebeling C, Wieder T, Sturm I, Daniel PT, Orfanos CE, Geilen CC (August 2001). "The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis". The Journal of Investigative Dermatology. 117 (2): 333–40. doi:10.1046/j.0022-202x.2001.01409.x. PMID 11511312.
- 1 2 Rieber M, Strasberg Rieber M (May 1998). "Induction of p53 without increase in p21WAF1 in betulinic acid-mediated cell death is preferential for human metastatic melanoma". DNA and Cell Biology. 17 (5): 399–406. doi:10.1089/dna.1998.17.399. PMID 9628583.
- 1 2 Selzer E, Pimentel E, Wacheck V, Schlegel W, Pehamberger H, Jansen B, Kodym R (May 2000). "Effects of betulinic acid alone and in combination with irradiation in human melanoma cells". The Journal of Investigative Dermatology. 114 (5): 935–40. doi:10.1046/j.1523-1747.2000.00972.x. PMID 10771474.
- ↑ Novel 3,28-Disubstituted Betulinic Acid Derivatives as Potent Anti-HIV Agents Aims/Hypothesis Out-licensing. iptechex pharmalicensing, IP Technology Exchange (2013)
- ↑ Uldis (2022-03-10). "How to use Betulinic acid in Cosmetics". NST Chemicals. Retrieved 2023-01-07.
- 1 2 Li, Jing; Zhang, Yansheng (June 19, 2014). "Modulating betulinic acid production in Saccharomyces cerevisiae by managing the intracellular supplies of the co-factor NADPH and oxygen". Journal of Bioscience and Bioengineering. 119 (1): 77–81. doi:10.1016/j.jbiosc.2014.06.013. PMID 25043336.
- ↑ Gauthier C, Legault J, Lebrun M, Dufour P, Pichette A (October 2006). "Glycosidation of lupane-type triterpenoids as potent in vitro cytotoxic agents". Bioorganic & Medicinal Chemistry. 14 (19): 6713–25. doi:10.1016/j.bmc.2006.05.075. PMID 16787747.