 | |
 | |
| Names | |
|---|---|
| Other names
bismuth(V) fluoride | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.029.205 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| BiF5 | |
| Molar mass | 303.97 g mol−1 |
| Appearance | long white needles,[1] colourless crystalline solid[2] |
| Density | 5.40 g cm−3[1] |
| Melting point | 151.4 °C (304.5 °F; 424.5 K) ,[2] 154.4 °C[1] |
| Boiling point | 230 °C (446 °F; 503 K)[1][2] |
| Structure | |
| octahedral Bi | |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H272, H314 | |
| P210, P220, P221, P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P370+P378, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | non-combustible |
| Safety data sheet (SDS) | MSDS |
| Related compounds | |
Other anions |
bismuth trichloride, bismuth tribromide, bismuth triiodide, pentamethylbismuth |
Other cations |
phosphorus pentafluoride, arsenic pentafluoride, antimony pentafluoride |
Related compounds |
bismuth trifluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Bismuth pentafluoride is an inorganic compound with the formula BiF5. It is a white solid that is highly reactive. The compound is of interest to researchers but not of particular value.
Structure
BiF5 is polymeric and consists of linear chains of trans-bridged corner sharing BiF6 octahedra.[1][3] This is the same structure as α-UF5.[1]
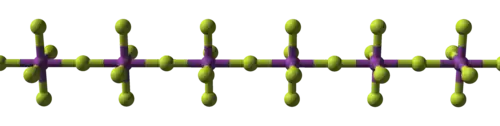  |  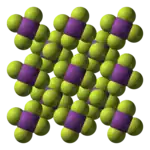 |
| (BiF5)∞ chain | packing of chains |
Preparation
BiF5 can be prepared by treating BiF3 with F2 at 500 °C.[2]
- BiF3 + F2 → BiF5
In an alternative synthesis, ClF3 is the fluorinating agent at 350 °C.[4]
- BiF3 + ClF3 → BiF5 + ClF
Reactions
Bismuth pentafluoride is the most reactive of the pnictogen pentafluorides and is an extremely strong fluorinating agent. It reacts vigorously with water to form ozone and oxygen difluoride, and with iodine or sulfur at room temperature. BiF5 fluorinates paraffin oil (hydrocarbons) to fluorocarbons above 50 °C and oxidises UF4 to UF6 at 150 °C. At 180 °C, bismuth pentafluoride fluorinates Br2 to BrF3 and Cl2 to ClF.[1]
BiF5 also reacts with alkali metal fluorides, MF, to form hexafluorobismuthates, M[BiF6], containing the hexafluorobismuthate anion, [BiF6]−.[2] Bismuth pentafluorude in hydrofluoric acid solvent also reacts with nickel fluoride to form the nickel salt of this anion, which can be incorporated into a complex with acetonitrile.[5]
References
- 1 2 3 4 5 6 7 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 561–563. ISBN 978-0-08-037941-8.
- 1 2 3 4 5 Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, pp. 769–770, ISBN 0-12-352651-5
- ↑ C. Hebecker (1971). "Zur Kristallstruktur von Wismutpentafluorid". Z. anorg. allg. Chem. 384 (2): 111–114. doi:10.1002/zaac.19713840204.
- ↑ A. I. Popov; A. V. Scharabarin; V. F. Sukhoverkhov; N. A. Tchumaevsky (1989). "Synthesis and properties of pentavalent antimony and bismuth fluorides". Z. Anorg. Allg. Chem. 576 (1): 242–254. doi:10.1002/zaac.19895760128.
- ↑ Roland Bougon; Pierrette Charpin; Karl O. Christe; Jacques Isabey; Monique Lance; Martine Nierlich; Julien Vigner; William W. Wilson (1988). "Preparation and characterization of nickel(2+) hexafluorobismuthate(1-) and of the ternary adducts [Ni(CH3CN)6](BiF6)2 and [Ni(CH3CN)6](SbF6)2. Crystal structure of hexakis(acetonitrile-d3)nickel(2+) hexafluoroantimonate". Inorganic Chemistry. 27 (8): 1389–1393. doi:10.1021/ic00281a018.
