 | |
| Names | |
|---|---|
| IUPAC name
Difluoro(dioxo)chromium | |
| Other names
Chromyl Fluoride, Chromium Difluoride Dioxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| EC Number |
|
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
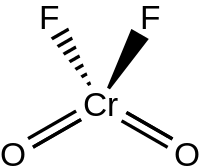
| CrO2F2 | |
| Molar mass | 121.991 g·mol−1 |
| Appearance | Violet-red crystals |
| Melting point | 31.6 °C (88.9 °F; 304.8 K) |
| Boiling point | 30 °C (86 °F; 303 K)[1] Sublimes |
| Structure | |
| monoclinic | |
| P21/c, No. 14 | |
| C2v | |
Formula units (Z) |
4 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Oxidant |
| Related compounds | |
Related compounds |
chromyl chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Chromyl fluoride is an inorganic compound with the formula CrO2F2. It is a violet-red colored crystalline solid that melts to an orange-red liquid.[2]
Structure
The liquid and gaseous CrO2F2 have a tetrahedral geometry with C2v symmetry, much like chromyl chloride.[3] Chromyl fluoride dimerizes via fluoride bridges (as O2Cr(μ-F)4CrO2) in the solid state, crystallizing in the P21/c space group with Z = 4. The Cr=O bond lengths are about 157 pm, and the Cr–F bond lengths are 181.7, 186.7, and 209.4 pm. Chromium resides in a distorted octahedral position with a coordination number of 6.[4]
History and preparation
Pure chromyl fluoride was first isolated in 1952 as reported by Alfred Engelbrecht and Aristid von Grosse.[5] It was first observed as red vapor in the early 19th century upon heating a mixture of fluorspar (CaF2), chromates, and sulfuric acid. These red vapors were initially thought to be CrF6, although some chemists assumed a CrO2F2 structure analogous to CrO2Cl2.[5] The first moderately successful synthesis of chromyl fluoride was reported by Fredenhagen who examined the reaction of hydrogen fluoride with alkali chromates. A later attempt saw von Wartenberg prepare impure CrO2F2 by treating chromyl chloride with elemental fluorine.[6] Another attempt was made by Wiechert, who treated HF with dichromate, yielding impure liquid CrO2F2 at −40 °C.
Engelbrecht and von Grosse's synthesis of CrO2F2, and most successive syntheses, involve treating chromium trioxide with a fluorinating agent:[5]
- CrO3 + 2 HF → CrO2F2 + H2O
The reaction is reversible, as water will readily hydrolyze CrO2F2 back to CrO3.
The approach published by Georg Brauer in the Handbook of Preparative Inorganic Chemistry[1] drew on von Wartenberg's approach[6] of direct fluoridation:
- CrO2Cl2 + F2 → CrO2F2 + Cl2
Other methods include treatment with chlorine fluoride, carbonyl fluoride, or some metal hexafluorides:
- CrO3 + 2 ClF → CrO2F2 + Cl2 + O2
- CrO3 + COF2 → CrO2F2 + CO2
- CrO3 + MF6 → CrO2F2 + MOF4 (M = Mo, W)
The last method involving the fluorides of tungsten and molybdenum are reported by Green and Gard to be very simple and effective routes to large quantities of pure CrO2F2.[2] They reported 100% yield when the reactions were conducted at 120 °C. As expected from the relative reactivities of MoF6 and WF6, the molybdenum reaction proceeded more readily than did the tungsten.[7]
Reactions
Chromyl fluoride is a strong oxidizing agent capable of converting hydrocarbons to ketones and carboxylic acids. It can also be used as a reagent in the preparation of other chromyl compounds.[2] Like some other fluoride compounds, CrO2F2 reacts with glass and quartz, so silicon-free plastics or metal containers are required for handling the compound. Its oxidizing power in inorganic systems has also been explored.[8] Chromyl fluoride can exchange fluorine atoms with metal oxides.
- CrO2F2 + MO → MF2 + CrO3
Chromyl fluoride will also convert the oxides of boron and silicon to the fluorides.[8]
Chromyl fluoride reacts with alkali and alkaline earth metal fluorides in perfluoroheptane (solvent) to produce orange-colored fluorochromates:[8]
- CrO2F2 + 2 MF → M2[CrO2F4]
Chromyl fluoride also reacts with Lewis acids, drawing carboxylate ligands from organic acid anhydrides and producing an acyl fluoride byproduct:[8]
- CrO2F2 + 2 (CF3CO)2O → (CF3COO)2CrO2 + 2 CF3COF
Chromyl fluoride forms adducts with weak bases NO, NO2, and SO2.
References
- 1 2 Brauer, Georg (1963) [1960]. "Chromyl Fluoride – CrO
2F
2". Handbook of Preparative Inorganic Chemistry, Volume 1 (2nd ed.). Stuttgart; New York: Ferdinand Enke Verlag; Academic Press, Inc. pp. 258–259. ISBN 978-0-32316127-5. - 1 2 3 Gard, G. L. (1986) "Chromium Difluoride Dioxide (Chromyl Fluoride)," Inorg. Synth., 24, 67-69, doi:10.1002/9780470132555.ch20.
- ↑ Hobbs, W. E. (1958) "Infrared Absorption Spectra of Chromyl Fluoride and Chromyl Chloride," J. Chem. Phys. 28(6), 1220-1222, doi:10.1063/1.1744372.
- ↑ Supeł, J.; Abram, U.; Hagenbach, A.; Seppelt, K. (2007) "Technetium Fluoride Trioxide, TcO3F, Preparation and Properties." Inorg. Chem., 46(14), 5591–5595, doi:10.1021/ic070333y.
- 1 2 3 Engelbrecht, A.; von Grosse, A. (1952) "Pure Chromyl Fluoride," J. Am. Chem. Soc. 74(21), 5262–5264, doi:10.1021/ja01141a007.
- 1 2 von Wartenberg, H. (1941) "Über höhere Chromfluoride (CrF
4, CrF
5 und CrO
2F
2)" [About higher chromium fluorides (CrF
4, CrF
5 and CrO
2F
2)], Z. Anorg. Allg. Chem. [in German], 247(1-2), 135–146, doi:10.1002/zaac.19412470112. - ↑ Green, P. J.; Gard, G. L. (1977) "Chemistry of Chromyl Fluoride. 5. New Preparative routes to CrO2F2," Inorg. Chem. 16(5), 1243–1245, doi:10.1021/ic50171a055.
- 1 2 3 4 Brown, S. D.; Green, P.J.; Gard, G.L. (1975) "The Chemistry of Chromyl Fluoride III: Reactions with Inorganic Systems," J. Fluorine Chem. 5(3), 203-219, doi:10.1016/S0022-1139(00)82482-3.