 | |
| Names | |
|---|---|
| IUPAC name
3,7-diazabicyclo[3.3.1]nonane | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H14N2 | |
| Molar mass | 126.203 g/mol |
| Melting point | 158-161 °C |
| Boiling point | 190-195 °C (9 Torr) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
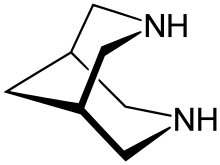
Bispidine (3,7-diazabicyclo[3.3.1]nonane) is an organic compound that is classified as a bicyclic diamine. Although synthetic, it is related structurally to natural alkaloid sparteine. It is a white crystalline solid. It has been widely investigated as a chelating agent. Many derivatives are known.
Structure and stereochemistry
Bispidine has a bicyclic scaffold consisting of two condensed piperidines. The unsubstituted bispidine backbone can assume three conformations: a chair-chair, boat-chair, and a boat-boat. In the gas phase, the chair-chair conformation predominates[1]
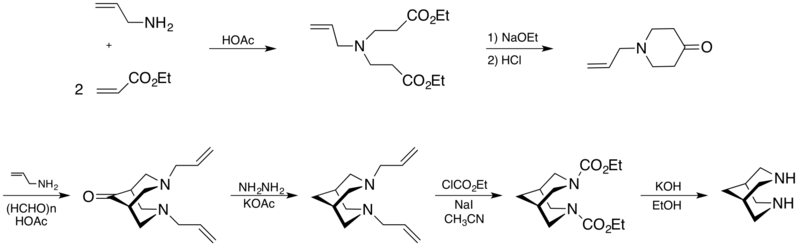
Synthesis
A convenient route to synthesize the bispidine molecule involves a pent-1-en-3-one and a prop-2-en-1-amine leading to a bis(carbethoxyethyl)allylamine which converts in 1-allylpiperidin-4-one by acid hydrolysis and decarboxylation. Then a Mannich reaction occurs, in particular a condensation of a 4-piperidone derivative with paraformaldehyde and allylamine in presence of acetic acid forms N,N’-diallylbispidin-9-one. This latter, results in the final bispidine after treatment with ethyl chloroformate in the presence of NaI, followed by alkaline hydrolysis.[2]
Reactions
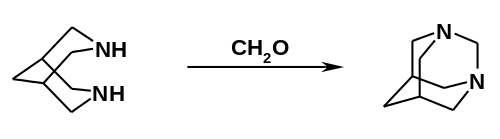
The reaction with formaldehyde gives the diazaadamantane.[3]
Bispidine Ligands
The bispidine unit can be chemically functionalized in several positions of its rigid bicyclic scaffold leading to a large number of bispidine-type ligands.[4]
Synthesis
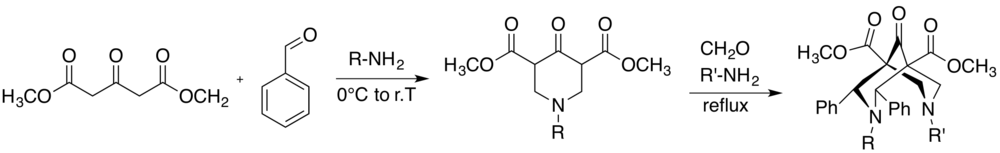
The first bispidine-based ligands dates back to 1930, when Carl Mannich reported the synthesis of two different substituted bispidine molecules.[5] The common route to afford the bispidine derivatives is a variation of the Mannich reaction, in particular two steps are required to afford the final product. The first step includes a reaction between a compound containing acidic C-H hydrogens, an aldehyde and a primary amine, using a predefined molar ratio of 1:2:1, respectively, leading to a piperidone. Additionally, a second condensation between the piperidone, an aliphatic aldehyde and a primary amine, using a ratio of 1:4:2, yields the desired bispidine. The reaction conditions need to be controlled to avoid the competitive aldol reaction.[6] Indeed the reaction solution should be as concentrated as possible to afford maximum yield, using alcohols, THF or other solvents listed in literature. Whereas high temperatures are preferred for one-step condensations, the two-step reaction is generally carried out at 0 °C for the first and refluxed during the second one.
Stereochemistry
Chemical substitutions on the bispidine backbone can influence the equilibrium of conformers. Many computational studies and others based on nuclear magnetic resonance (1H and 13C NMR), X-ray crystallography and Raman spectroscopy have been done to investigate the different conformational entities of bispidine derivatives.[7]
Coordination Chemistry
Bispidine-based ligands have been mostly employed in coordination chemistry.[4] The first transition metal complex with bidentate bispidine dates back to 1957.[8] Indeed, by the addition of further metal binding sites in the basic bispidine scaffold, that has already two aliphatic aminic N donor atoms, efficient stable metal complexes can be selectively synthesized, thus obtaining tetra-,[9] penta-,[10] hexa-[11] and octa-[12] dentate species.
Potential applications
Because of the chemical versatility of the bispidine scaffold and due to the metal selectivity and complexes stability of these type of ligand, they have been proposed for many applications, although none have been commercialized
Catalysis
Cu(II)-bispidine complexes catalyze aziridations.[13] Amino acids-modified bispidine framework support catalysts for the enantio-selective aldol reaction of functionalized ketones.[14] Bispidine-based iron complexes have been investigated for the oxidation of olefin and unactivated C-H bonds.[15]
Pharmaceutical use
Several patents relate to the use of bispidine ligands for their antiarrhythmic[16] and analgesic activity.[17] It has been also found out that they exhibit high affinity and selectivity to ĸ-opioid receptors and many studies concerning the influence of structural variation towards their biological activity were also reported.[18]
Medicine
Bispidine systems were also employed as bifunctional chelators for PET exams.[19] Fast complexation, availability of different functionalities for the linking to the targeting vectors, and a cost-effective way to synthesize them in the multigram scale are among these requirements. The functionalization and optimization of pentadentate bispidine derivatives and the evaluation of the potential of radiocopper−bispidine complexes as PET tracers have been done.
Other
Bispidine derivatives have been used as ligands to build novel one-dimensional coordination polymers, showing an interesting influence on the dynamic behaviour of these hybrid systems.[20]
References
- ↑ Mastryukov, V.S.; Osina, E. L.; Dorofeeva, O. V.; Popik, M. V.; Vilkov, L. V.; Belikova, N. A. (1979). "An electron diffraction study of the molecular structure of gaseous bicyclo[3.3.1]nonane". J. Mol. Struct. 52 (1): 211–224. Bibcode:1979JMoSt..52..211M. doi:10.1016/0022-2860(79)80119-2.
- ↑ Miyahara, Y.; Goto, K.; Inazu, T. (2001). "Convenient Synthesis of 3,7-Diazabicyclo[3.3.1]nonane (Bispidine)". Synthesis. 2001 (3): 364–366. doi:10.1055/s-2001-11427.
- ↑ Galinovsky, F.; Langer, H. (1955). "Synthese des 1,3-Diaza-adamantans und des Bispidins". Monatshefte für Chemie. 86 (3): 449–453. doi:10.1007/BF00903631.
- 1 2 Comba, P.; Kerscher, M.; Schiek, W. (2005). "Bispidine Coordination Chemistry". Progress in Inorganic Chemistry. 55 (Chapter 9): 613–704. doi:10.1002/9780470144428.ch9.
- ↑ Mannich, C.; Mohs, P. (1930). "Über Derivate eines aus zwei Piperidinringen kondensierten bicyclischen Systems". Chem. Ber. 63 (3): 608–612. doi:10.1002/cber.19300630314.
- ↑ Holzgrabe, U.; Ericyas, E. (1992). "Synthese und Stereochemie potentiell stark analgetischer 2,4‐m‐diarylsubstituierter 3,7‐Diazabicyclo[3.3.1]nonan‐9‐on‐1,5‐diester". Archiv der Pharmazie. 325 (10): 657–663. doi:10.1002/ardp.19923251008. PMID 1334646.
- ↑ Jeyaraman, R.; Avila, S. (1981). "Chemistry of 3-Azabicyclo[3.3.1]nonanes". Chemical Reviews. 81 (2): 149–174. doi:10.1021/cr00042a002.
- ↑ Stetter, H.; Merten, R. (1957). "Über Verbindungen mit Urotropin‐Struktur, IX. Zur Kenntnis des Bispidins". Chem. Ber. 90 (6): 868–875. doi:10.1002/cber.19570900605.
- ↑ Comba, P.; Kanellakopulos, B.; Katsichtis, C.; Lienke, A.; Pritzkow, H.; Rominger, F. (1998). "Synthesis and characterisation of manganese(II) compounds with tetradentate ligands based on the bispidine backbone". Journal of the Chemical Society, Dalton Transactions (23): 3997–4002. doi:10.1039/A805944F.
- ↑ Comba, P.; Kerscher, M.; Lawrance, G.A.; Martin, B.; Wadepohl, H.; Wunderlich, S. (2008). "Stable Five‐ and Six‐Coordinate Cobalt(III) Complexes with a Pentadentate Bispidine Ligand†". Angew. Chem. Int. Ed. 47 (25): 4740–4743. doi:10.1002/anie.200800515. PMID 18484579.
- ↑ Bleiholder, C.; Börzel, H.; Comba, P.; Ferrari, R.; Heydt, M.; Kerscher, M.; Kuwata, S.; Laurenczy, G.; Lawrance, G.A.; Lienke, A.; Martin, B.; Merz, M.; Nuber, B.; Pritzkow, H. (2005). "Coordination Chemistry of a New Rigid, Hexadentate Bispidine-Based Bis(amine)tetrakis(pyridine) Ligand". Inorg. Chem. 44 (22): 8145–8155. doi:10.1021/ic0513383. PMID 16241165.
- ↑ Comba, P.; Jermilova, U.; Orvig, C.; Patrick, B.O.; Ramogida, C.F.; Reck, K.; Schneider, C.; Starke, M. (2017). "Octadentate Picolinic Acid‐Based Bispidine Ligand for Radiometal Ions". Chem. Eur. J. 23 (63): 15945–15956. doi:10.1002/chem.201702284. PMID 28815804.
- ↑ Comba, P.; Merz, M.; Pritzkow, H. (2003). "Catalytic Aziridination of Styrene with Copper Complexes of Substituted 3,7‐Diazabicyclo[3.3.1]nonanones". Eur. J. Inorg. Chem. 2003 (9): 1711–1718. doi:10.1002/ejic.200200618.
- ↑ Liu, J.; Yang, Z.; Wang, Z.; Wang, F.; Chen, X.; Liu, X.; Feng, X.; Su, Z.; Hu, C. (2008). "Asymmetric Direct Aldol Reaction of Functionalized Ketones Catalyzed by Amine Organocatalysts Based on Bispidine". J. Am. Chem. Soc. 130 (17): 5654–5655. doi:10.1021/ja800839w. PMID 18380434.
- ↑ Bautz, J.; Comba, P.; Lopez de Laorden, C.; Menzel, M.; Rajaraman, G. (2007). "Biomimetic High‐Valent Non‐Heme Iron Oxidants for the cis‐Dihydroxylation and Epoxidation of Olefins†". Angew. Chem. Int. Ed. 46 (42): 8067–8070. doi:10.1002/anie.200701681. PMID 17868164.
- ↑ Ruenitz, P.C.; Mokler, C.M. (1997). "Analogues of Sparteine. 5. Antiarrhythmic Activity of Selected , '-Disubstituted Bispidines". J. Med. Chem. 20 (12): 1668–1671. doi:10.1021/jm00222a026. PMID 592332.
- ↑ Samhammer, A.; Holzgrabe, U.; Haller, R. (1989). "Synthese, Stereochemie und analgetische Wirkung von 3,7‐Diazabicyclo[3.3.1]nonan‐9‐onen und 1,3‐Diazaadamantan‐6‐onen)". Archiv der Pharmazie. 322 (9): 551–555. doi:10.1002/ardp.19893220908. PMID 2610588.
- ↑ Siener, T.; Holzgrabe, U.; Drosihn, S.; Brandt, W. (1999). "Conformational and configurational behaviour of κ-agonistic 3,7-diazabicyclo[3.3.1]nonan-9-ones—synthesis, nuclear magnetic resonance studies and semiempirical PM3 calculations". Journal of the Chemical Society, Perkin Transactions 2. 2 (9): 1827–1834. doi:10.1039/A806641H.
- ↑ Comba, P.; Kubeil, M.; Pietzsch, J.; Rudolf, H.; Stephan, H.; Zarschler, K. (2014). "Bispidine Dioxotetraaza Macrocycles: A New Class of Bispidines for 64Cu PET Imaging". Inorganic Chemistry. 53 (13): 6698–6707. doi:10.1021/ic500476u. PMID 24906110.
- ↑ Rossetti, A.; Lippi, M.; Marti-Rujas, J.; Sacchetti, A.; Canetti, M. (2018). "Highly Dynamic and Tunable Behavior of 1D Coordination Polymers based on the Bispidine Ligand". Chem. Eur. J. 24 (72): 19368–19372. doi:10.1002/chem.201804782. PMID 30325090.