 | |
| Clinical data | |
|---|---|
| Trade names | Brenzavvy, Bexacat |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
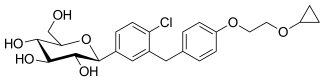
| Formula | C24H29ClO7 |
| Molar mass | 464.94 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Bexagliflozin, sold under the brand name Brenzavvy, is an antidiabetic medication used to improve glycemic control in adults with type 2 diabetes as an adjunct to diet and exercise.[4] It is a sodium-glucose cotransporter 2 (SGLT2) inhibitor that is taken by mouth.[1][2]
Medical uses
Bexagliflozin is indicated to improve glycemic control in adults with type 2 diabetes as an adjunct to diet and exercise.[3][4]
Research
A 96-week phase II clinical study of adults with type 2 diabetes showed that bexagliflozin monotherapy provided a durable, clinically meaningful improvement of glycemic control, with a substantial reduction in weight and blood pressure, but no increase in the rate of significant adverse events.[5][6] In a clinical study of patients with type 2 diabetes and stage 3a/3b chronic kidney disease, bexagliflozin was well tolerated and shown to reduce hemoglobin A1c levels, body weight, systolic blood pressure and albuminuria.[7]
Veterinary uses
The data from two six-month field studies and an extended use field study demonstrated that bexagliflozin was over 80% effective in improving glycemic control in cats with diabetes mellitus.[2]
Bexagliflozin, sold under the brand name Bexacat, is an antidiabetic medication used to improve glycemic control in cats with diabetes.[2] Bexacat is the first sodium-glucose cotransporter 2 (SGLT2) inhibitor new animal drug approved by the US Food and Drug Administration (FDA) in any animal species.[2] It was approved for medical use in the United States in December 2022.[2][8] Bexacat is sponsored by Increvet Inc., based in Boston, Massachusetts.[2] Elanco licensed development and commercialization rights for bexagliflozin from Bexcafe, an affiliate of Increvet.[8]
References
- 1 2 "Bexacat- bexagliflozin tablets tablet". DailyMed. 16 January 2023. Archived from the original on 21 January 2023. Retrieved 21 January 2023.
- 1 2 3 4 5 6 7 "FDA Approves First Oral Treatment for Cats with Diabetes Mellitus". U.S. Food and Drug Administration (FDA). 8 December 2022. Archived from the original on 11 December 2022. Retrieved 11 December 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 "Brenzavvy- bexagliflozin tablet". DailyMed. 18 September 2023. Archived from the original on 20 November 2023. Retrieved 20 November 2023.
- 1 2 "Novel Drug Approvals for 2023". U.S. Food and Drug Administration (FDA). 20 January 2023. Archived from the original on 21 January 2023. Retrieved 21 January 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ Halvorsen YD, Lock JP, Frias JP, Tinahones FJ, Dahl D, Conery AL, et al. (September 2022). "A 96-week, double-blind, randomized controlled trial comparing bexagliflozin to glimepiride as an adjunct to metformin for the treatment of type 2 diabetes in adults". Diabetes, Obesity and Metabolism. 25 (1): 293–301. doi:10.1111/dom.14875. PMID 36178197. S2CID 252623503.
{{cite journal}}: CS1 maint: overridden setting (link) - ↑ Halvorsen YC, Walford GA, Massaro J, Aftring RP, Freeman MW (November 2019). "A 96-week, multinational, randomized, double-blind, parallel-group, clinical trial evaluating the safety and effectiveness of bexagliflozin as a monotherapy for adults with type 2 diabetes". Diabetes, Obesity and Metabolism. 21 (11): 2496–2504. doi:10.1111/dom.13833. PMID 31297965. S2CID 195892291.
- ↑ Allegretti AS, Zhang W, Zhou W, Thurber TK, Rigby SP, Bowman-Stroud C, et al. (September 2019). "Safety and effectiveness of bexagliflozin in patients with type 2 diabetes mellitus and stage 3a/3b CKD". American Journal of Kidney Diseases. 74 (3): 328–337. doi:10.1053/j.ajkd.2019.03.417. PMC 10077840. PMID 31101403. S2CID 157066958.
{{cite journal}}: CS1 maint: overridden setting (link) - 1 2 "Elanco Announces FDA Approval of Bexacat (bexagliflozin tablets) – the First-of-its-Kind Oral Feline Diabetes Treatment Option" (Press release). Elanco. 9 December 2022. Archived from the original on 11 December 2022. Retrieved 11 December 2022.
Further reading
- Benedict SL, Mahony OM, McKee TS, Bergman PJ (January 2022). "Evaluation of bexagliflozin in cats with poorly regulated diabetes mellitus". Canadian Journal of Veterinary Research. 86 (1): 52–58. PMC 8697324. PMID 34975223.
- Zhang W, Li X, Ding H, Lu Y, Stilwell GE, Halvorsen YD, et al. (May 2020). "Metabolism and disposition of the SGLT2 inhibitor bexagliflozin in rats, monkeys and humans". Xenobiotica. 50 (5): 559–569. doi:10.1080/00498254.2019.1654634. PMID 31432741. S2CID 201115043.
{{cite journal}}: CS1 maint: overridden setting (link)