Calmodulin 2 is a protein that in humans is encoded by the CALM2 gene.[3][4] A member of the calmodulin family of signaling molecules, it is an intermediary between calcium ions, which act as a second messenger, and many intracellular processes, such as the contraction of cardiac muscle.[5]
Clinical significance
Mutations in CALM2 are associated with cardiac arrhythmias.[6] In particular, several single-nucleotide polymorphisms of CALM2 have been reported as potential causes of sudden infant death syndrome. Due to their heritability, CALM2 mutations can affect multiple children in a family,[7] and the discovery of the deadly consequences of these mutations has led to challenges against the murder convictions of mothers of multiple deceased infants, as in the case of Kathleen Folbigg, acquitted after more than 20 years imprisonment, in Australia.[8]
Interactions
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000143933 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Entrez Gene: CALM2 Calmodulin 2 (phosphorylase kinase, delta)".
- ↑ SenGupta B, Friedberg F, Detera-Wadleigh SD (December 1987). "Molecular analysis of human and rat calmodulin complementary DNA clones. Evidence for additional active genes in these species". The Journal of Biological Chemistry. 262 (34): 16663–16670. doi:10.1016/S0021-9258(18)49306-4. PMID 2445749.
- ↑ "Entry - *114205 - CALCIUM CHANNEL, VOLTAGE-DEPENDENT, L TYPE, ALPHA-1C SUBUNIT; CACNA1C - OMIM". omim.org. Retrieved 2023-06-01.
- ↑ Makita N, Yagihara N, Crotti L, Johnson CN, Beckmann BM, Roh MS, et al. (August 2014). "Novel calmodulin mutations associated with congenital arrhythmia susceptibility". Circulation: Cardiovascular Genetics. 7 (4): 466–474. doi:10.1161/CIRCGENETICS.113.000459. PMC 4140998. PMID 24917665.
- ↑ Brohus M, Arsov T, Wallace DA, Jensen HH, Nyegaard M, Crotti L, et al. (March 2021). "Infanticide vs. inherited cardiac arrhythmias". Europace. Volume 23, Issue 3. European Heart Rhythm Association (published 17 November 2020). 23 (3): 441–450. doi:10.1093/europace/euaa272. PMC 7947592. PMID 33200177.
- ↑ Schwartz O. "4 Dead Infants, a Convicted Mother, and a Genetic Mystery". Wired. ISSN 1059-1028. Retrieved 2023-06-01.
- ↑ Takahashi M, Yamagiwa A, Nishimura T, Mukai H, Ono Y (September 2002). "Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring gamma-tubulin ring complex". Molecular Biology of the Cell. 13 (9): 3235–3245. doi:10.1091/mbc.E02-02-0112. PMC 124155. PMID 12221128.
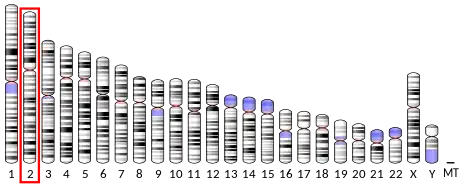
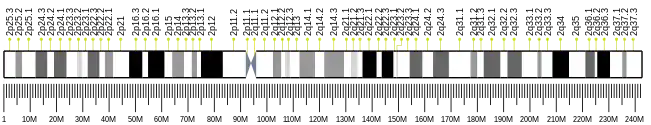
- ↑ Berchtold MW, Egli R, Rhyner JA, Hameister H, Strehler EE (May 1993). "Localization of the human bona fide calmodulin genes CALM1, CALM2, and CALM3 to chromosomes 14q24-q31, 2p21.1-p21.3, and 19q13.2-q13.3". Genomics. 16 (2): 461–465. doi:10.1006/geno.1993.1211. PMID 8314583.
External links
- Human CALM2 genome location and CALM2 gene details page in the UCSC Genome Browser.
Further reading
- Zhang M, Yuan T (1998). "Molecular mechanisms of calmodulin's functional versatility". Biochemistry and Cell Biology. 76 (2–3): 313–323. doi:10.1139/bcb-76-2-3-313. PMID 9923700.
- Gusev NB (October 2001). "Some properties of caldesmon and calponin and the participation of these proteins in regulation of smooth muscle contraction and cytoskeleton formation". Biochemistry. Biokhimiia. 66 (10): 1112–1121. doi:10.1023/A:1012480829618. PMID 11736632. S2CID 310781.
- Benaim G, Villalobo A (August 2002). "Phosphorylation of calmodulin. Functional implications". European Journal of Biochemistry. 269 (15): 3619–3631. doi:10.1046/j.1432-1033.2002.03038.x. hdl:10261/79981. PMID 12153558.
- Koller M, Schnyder B, Strehler EE (October 1990). "Structural organization of the human CaMIII calmodulin gene". Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 1087 (2): 180–189. doi:10.1016/0167-4781(90)90203-e. PMID 2223880.
- Baudier J, Mochly-Rosen D, Newton A, Lee SH, Koshland DE, Cole RD (May 1987). "Comparison of S100b protein with calmodulin: interactions with melittin and microtubule-associated tau proteins and inhibition of phosphorylation of tau proteins by protein kinase C". Biochemistry. 26 (10): 2886–2893. doi:10.1021/bi00384a033. PMID 3111527.
- Matoba R, Okubo K, Hori N, Fukushima A, Matsubara K (September 1994). "The addition of 5'-coding information to a 3'-directed cDNA library improves analysis of gene expression". Gene. 146 (2): 199–207. doi:10.1016/0378-1119(94)90293-3. PMID 8076819.
- Srinivas SK, Srinivas RV, Anantharamaiah GM, Compans RW, Segrest JP (October 1993). "Cytosolic domain of the human immunodeficiency virus envelope glycoproteins binds to calmodulin and inhibits calmodulin-regulated proteins". The Journal of Biological Chemistry. 268 (30): 22895–22899. doi:10.1016/S0021-9258(18)41610-9. PMID 8226798.
- Miller MA, Mietzner TA, Cloyd MW, Robey WG, Montelaro RC (November 1993). "Identification of a calmodulin-binding and inhibitory peptide domain in the HIV-1 transmembrane glycoprotein". AIDS Research and Human Retroviruses. 9 (11): 1057–1066. doi:10.1089/aid.1993.9.1057. PMID 8312049.
- Radding W, Pan ZQ, Hunter E, Johnston P, Williams JP, McDonald JM (January 1996). "Expression of HIV-1 envelope glycoprotein alters cellular calmodulin". Biochemical and Biophysical Research Communications. 218 (1): 192–197. doi:10.1006/bbrc.1996.0034. PMID 8573130.
- Pan Z, Radding W, Zhou T, Hunter E, Mountz J, McDonald JM (September 1996). "Role of calmodulin in HIV-potentiated Fas-mediated apoptosis". The American Journal of Pathology. 149 (3): 903–910. PMC 1865159. PMID 8780394.
- Sasaki M, Uchiyama J, Ishikawa H, Matsushita S, Kimura G, Nomoto K, Koga Y (October 1996). "Induction of apoptosis by calmodulin-dependent intracellular Ca2+ elevation in CD4+ cells expressing gp 160 of HIV". Virology. 224 (1): 18–24. doi:10.1006/viro.1996.0502. PMID 8862395.
- Brigino E, Haraguchi S, Koutsonikolis A, Cianciolo GJ, Owens U, Good RA, Day NK (April 1997). "Interleukin 10 is induced by recombinant HIV-1 Nef protein involving the calcium/calmodulin-dependent phosphodiesterase signal transduction pathway". Proceedings of the National Academy of Sciences of the United States of America. 94 (7): 3178–3182. Bibcode:1997PNAS...94.3178B. doi:10.1073/pnas.94.7.3178. PMC 20342. PMID 9096366.
- Minakami R, Jinnai N, Sugiyama H (August 1997). "Phosphorylation and calmodulin binding of the metabotropic glutamate receptor subtype 5 (mGluR5) are antagonistic in vitro". The Journal of Biological Chemistry. 272 (32): 20291–20298. doi:10.1074/jbc.272.32.20291. PMID 9242710.
- Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY (August 1997). "Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin". Nature. 388 (6645): 882–887. doi:10.1038/42264. PMID 9278050. S2CID 13745050.
- Malvoisin E, Wild F (August 1997). "Inhibition of HIV-1, HIV-2 and SIV envelope glycoprotein-mediated cell fusion by calmodulin". Virus Research. 50 (2): 119–127. doi:10.1016/S0168-1702(97)00060-9. PMID 9282777.
- Martoglio B, Graf R, Dobberstein B (November 1997). "Signal peptide fragments of preprolactin and HIV-1 p-gp160 interact with calmodulin". The EMBO Journal. 16 (22): 6636–6645. doi:10.1093/emboj/16.22.6636. PMC 1170268. PMID 9362478.
- Ishikawa H, Sasaki M, Noda S, Koga Y (August 1998). "Apoptosis induction by the binding of the carboxyl terminus of human immunodeficiency virus type 1 gp160 to calmodulin". Journal of Virology. 72 (8): 6574–6580. doi:10.1128/JVI.72.8.6574-6580.1998. PMC 109834. PMID 9658102.
- Toutenhoofd SL, Foletti D, Wicki R, Rhyner JA, Garcia F, Tolon R, Strehler EE (May 1998). "Characterization of the human CALM2 calmodulin gene and comparison of the transcriptional activity of CALM1, CALM2 and CALM3". Cell Calcium. 23 (5): 323–338. doi:10.1016/S0143-4160(98)90028-8. PMID 9681195.










![1qiv: CALMODULIN COMPLEXED WITH N-(3,3,-DIPHENYLPROPYL)-N'-[1-R-(3,4-BIS-BUTOXYPHENYL)-ETHYL]-PROPYLENEDIAMINE (DPD), 1:2 COMPLEX](../I/PDB_1qiv_EBI.jpg.webp)
![1qiw: CALMODULIN COMPLEXED WITH N-(3,3,-DIPHENYLPROPYL)-N'-[1-R-(3,4-BIS-BUTOXYPHENYL)-ETHYL]-PROPYLENEDIAMINE (DPD)](../I/PDB_1qiw_EBI.jpg.webp)