The Cahn–Hilliard equation (after John W. Cahn and John E. Hilliard)[1] is an equation of mathematical physics which describes the process of phase separation, by which the two components of a binary fluid spontaneously separate and form domains pure in each component. If is the concentration of the fluid, with indicating domains, then the equation is written as
where is a diffusion coefficient with units of and gives the length of the transition regions between the domains. Here is the partial time derivative and is the Laplacian in dimensions. Additionally, the quantity is identified as a chemical potential.
Related to it is the Allen–Cahn equation, as well as the stochastic Cahn–Hilliard Equation and the stochastic Allen–Cahn equation.
Features and applications
Of interest to mathematicians is the existence of a unique solution of the Cahn–Hilliard equation, given by smooth initial data. The proof relies essentially on the existence of a Lyapunov functional. Specifically, if we identify
as a free energy functional, then
so that the free energy does not grow in time. This also indicates segregation into domains is the asymptotic outcome of the evolution of this equation.
In real experiments, the segregation of an initially mixed binary fluid into domains is observed. The segregation is characterized by the following facts.
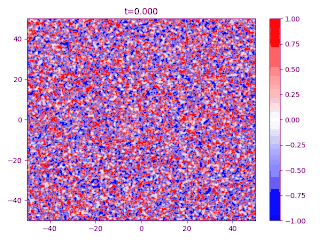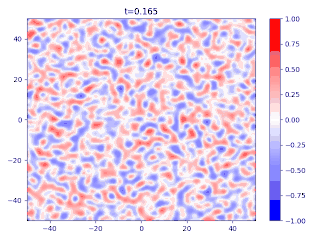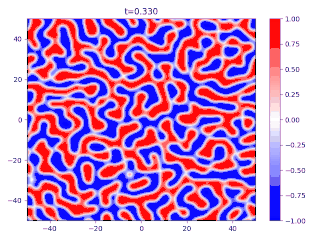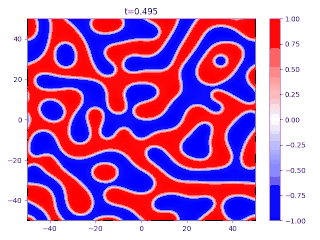
- There is a transition layer between the segregated domains, with a profile given by the function and hence a typical width because this function is an equilibrium solution of the Cahn–Hilliard equation.
- Of interest also is the fact that the segregated domains grow in time as a power law. That is, if is a typical domain size, then . This is the Lifshitz–Slyozov law, and has been proved rigorously for the Cahn–Hilliard equation and observed in numerical simulations and real experiments on binary fluids.
- The Cahn–Hilliard equation has the form of a conservation law, with . Thus the phase separation process conserves the total concentration , so that .
- When one phase is significantly more abundant, the Cahn–Hilliard equation can show the phenomenon known as Ostwald ripening, where the minority phase forms spherical droplets, and the smaller droplets are absorbed through diffusion into the larger ones.
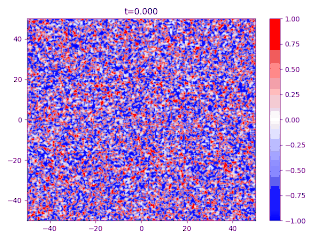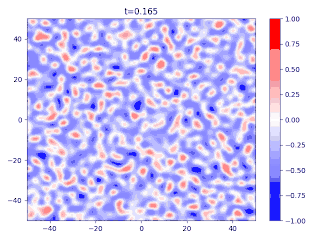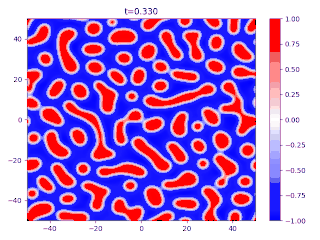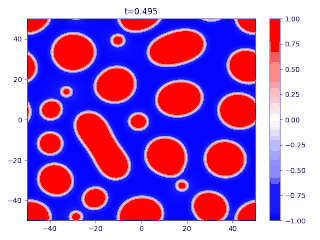 Evolution of random initial data with and (60/40 mix of the blue and red phases, respectively), demonstrating Ostwald ripening
Evolution of random initial data with and (60/40 mix of the blue and red phases, respectively), demonstrating Ostwald ripening
The Cahn–Hilliard equations finds applications in diverse fields: in complex fluids and soft matter (interfacial fluid flow, polymer science and in industrial applications). The solution of the Cahn–Hilliard equation for a binary mixture demonstrated to coincide well with the solution of a Stefan problem and the model of Thomas and Windle.[2] Of interest to researchers at present is the coupling of the phase separation of the Cahn–Hilliard equation to the Navier–Stokes equations of fluid flow.
See also
Further reading
- Cahn, John W.; Hilliard, John E. (1958). "Free Energy of a Nonuniform System. I. Interfacial Free Energy". The Journal of Chemical Physics. AIP Publishing. 28 (2): 258–267. Bibcode:1958JChPh..28..258C. doi:10.1063/1.1744102. ISSN 0021-9606.
- Bray, A.J. (1994). "Theory of phase-ordering kinetics". Advances in Physics. 43 (3): 357–459. arXiv:cond-mat/9501089. Bibcode:1994AdPhy..43..357B. doi:10.1080/00018739400101505. ISSN 0001-8732. S2CID 83182.
- Zhu, Jingzhi; Chen, Long-Qing; Shen, Jie; Tikare, Veena (1999-10-01). "Coarsening kinetics from a variable-mobility Cahn-Hilliard equation: Application of a semi-implicit Fourier spectral method". Physical Review E. American Physical Society (APS). 60 (4): 3564–3572. Bibcode:1999PhRvE..60.3564Z. doi:10.1103/physreve.60.3564. ISSN 1063-651X. PMID 11970189.
- Elliott, Charles M.; Songmu, Zheng (1986). "On the Cahn-Hilliard equation". Archive for Rational Mechanics and Analysis. Springer Nature. 96 (4): 339–357. Bibcode:1986ArRMA..96..339E. doi:10.1007/bf00251803. ISSN 0003-9527. S2CID 56206640.
- Areias, P.; Samaniego, E.; Rabczuk, T. (2015-12-17). "A staggered approach for the coupling of Cahn–Hilliard type diffusion and finite strain elasticity". Computational Mechanics. Springer Science and Business Media LLC. 57 (2): 339–351. doi:10.1007/s00466-015-1235-1. ISSN 0178-7675. S2CID 123982946.
- Hashimoto, Takeji; Matsuzaka, Katsuo; Moses, Elisha; Onuki, Akira (1995-01-02). "String Phase in Phase-Separating Fluids under Shear Flow". Physical Review Letters. American Physical Society (APS). 74 (1): 126–129. Bibcode:1995PhRvL..74..126H. doi:10.1103/physrevlett.74.126. ISSN 0031-9007. PMID 10057715.
- T. Ursell, “Cahn–Hilliard Kinetics and Spinodal Decomposition in a Diffuse System,” California Institute of Technology (2007).
References
- ↑ Cahn, John W.; Hilliard, John E. (February 1958). "Free Energy of a Nonuniform System. I. Interfacial Free Energy". The Journal of Chemical Physics. 28 (2): 258–267. Bibcode:1958JChPh..28..258C. doi:10.1063/1.1744102. ISSN 0021-9606.
- ↑ Vermolen, F. J.; Gharasoo, M. G.; Zitha, P. L. J.; Bruining, J. (2009). "Numerical Solutions of Some Diffuse Interface Problems: The Cahn–Hilliard Equation and the Model of Thomas and Windle". International Journal for Multiscale Computational Engineering. 7 (6): 523–543. doi:10.1615/IntJMultCompEng.v7.i6.40.