Calcium (ion) batteries are energy storage and delivery technologies (i.e., electro–chemical energy storage) that employ calcium ions (cations), Ca2+, as the active charge carrier in the electrolytes as well as in the electrodes (anode and cathode).[1][2][3] Calcium (ion) batteries remain an active area of research,[4][5] with studies and work persisting in the discovery and development of electrodes and electrolytes that enable stable, long-term battery operation.[6]
History
The application of calcium batteries date back to the 1960s in thermal batteries for military and space applications.[7] The first example of an electrochemical cell was Ca//SOCl2 as a primary cell.[8] Early examination of Ca2+ intercalation hosts proposed transition metal oxides and sulfides.[9] The study of calcium batteries as well as calcium electro-chemistry has continued since then, and has seen expanded research owing to recent developments in effective Ca-metal redox activity, particularly at room temperature, which had been a longstanding challenge in the field.
Benefits and advantages
Material properties
In terms of inherent materials properties, calcium metal is known for its high conductivity and very high melting temperature (842 °C) relative to other metals. The higher melting temperature can make calcium metal inherently safer. Calcium is also an environmentally benign element, mitigating concerns over toxicity.
Resource and supply
Calcium batteries are considered a next generation battery or post-Li-ion battery energy storage system, namely one of the many candidates that may potentially replace lithium-ion battery technology. It is also a multivalent battery. Key advantages are lower cost, earth abundance (41,500 ppm), higher energy density, by a combination of high capacity and a high cell voltage,[10] and potentially higher power output. Calcium is the 5th most abundant mineral in the Earth's crust, and the most abundant alkaline earth metal, and the third most abundant metal after aluminum (Al) and iron (Fe).[11] The United States is the largest producer (by annual production) of calcium sources (primarily lime & egg shells), promising domestic supply and manufacture. Other major producers include Russia and China.
Electrochemistry
As compared to other divalent systems, calcium batteries have a possibility of higher cell voltages than magnesium batteries due to the 0.5 V lower standard reduction potential of the former. Ca2+ ions also have the potential for faster reaction kinetics as compared to magnesium (Mg2+) owing to its less polarizing properties and charge density both in the electrolyte as well as in an intercalation cathode.
Capacity and energy density
Calcium metal anodes have a 2+ oxidation state which would provide a greater energy density over monovalent systems (i.e., Li+ and Na+), and it has a standard reduction potential of 2.9 V, which is only 0.17 V greater than that of lithium metal. A calcium metal anode offers a higher volumetric capacity and gravimetric capacities (2072 mAh.mL−1 and 1337 mAh.g−1, respectively) than current commercial graphite anodes in Li-ion batteries (300–430 mAh mL−1 and 372 mAh g−1).[12] A calcium sulfur (Ca//S) battery has theoretical energy densities of 3202 Wh/L and 1835 Wh/kg, versus 2800 Wh/L for Li//S.
Battery components
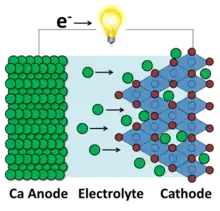
Currently, a calcium (ion) battery has yet to be commercialized, but remains in the realm of research and development. Efforts concentrate on developing effective anode and cathode materials, as well as stable electrolytes. Intensive focus has been placed on achieving reliable electrochemistry with a pure calcium metal anode in order to achieve high operating voltages, capacities, and energy densities. However, carbon and metal oxide based anodes, while providing lower performance metrics, are also reliable. Cathodes has sought to achieve high Ca2+ migration kinetics, high capacity, as well as high operative voltages.
Electrolytes
Calcium salt component
Salts explored thus far in liquid electrolytes include: calcium tetrafluoroborate (Ca(BF4)2, calcium borohydride (Ca(BH4)2, calcium bis(trifluoromethanesulfonimide) (Ca(TFSI)2), calcium perchlorate (Ca(ClO4)2), calcium hexafluorophosphate (Ca(PF6)2), and calcium nitrate (Ca(NO3)2). Calcium nitrate is commonly used in aqueous batteries. Early studies revealed that reversible Ca deposition using simple Ca salts are impossible at room temperature. A Ca salt using a bulky low-coordinating tetra-hexafluoroisopropoxy borate anion [Ca(B(Ohfip)4)2] have been examined by three research groups independently and shown to be active for Ca deposition at room temperature with the Coulumbic efficiency up to 80% and anodic stability up to 4.1 V vs Ca.[13][14][15] The [Ca(B(Ohfip)4)2] electrolyte remains to be the most active electrolyte for Ca deposition, but still far below the standard for practical applications.
Liquid electrolytes
Several different electrolyte systems have been examined for calcium (ion) batteries. Electrolytes are still an area of investigation, where previous work has shown that many show low electrochemical stability. Redox reactions on calcium metal in several organic electrolytes was initially examined and concluded no Ca deposition using (Ca(ClO4)2) and Ca(BF4)2 in organic solvents.[16] Water as the electrolyte has been examined in a calcium ion battery.[17] Alkyl carbonate electrolyte have also been examined.[18][19] Theoretical studies have also been conducted on both salts and aprotic solvents showing on favorable solvation/de-solvation properties.[20][21] This has also been followed by experimental observations of salt solvation by different solvents.[22] Ionic liquids have also been examined.[23] Mixed cation electrolytes with Li/Ca and Na/Ca (BH4- and PF6- anion) have been examined with promising solvent and SEI properties.[24][25]
Polymer electrolytes
Polymer electrolytes have also been examined to provide the combined functions as both the battery separator and the electrolyte. One of the first samples of a polymer electrolyte was PVA/PVP complexed with CaCl2.[26] Subsequent studies demonstrated polymer electrolytes made from poly(ethylene glycol) diacrylate (PEDGA)[27] and polytetrahydrofuran (PTHF)[28] both with calcium nitrate (Ca(NO3)2), polyethylene oxide,[29] single-ion conducting polymers based on PEG and PTHF backbones and TFSI anions,[30] and PEDGA-based gel polymer electrolytes, using such solvents as alkyl carbonates and ionic liquid solvents.[31][32] Most recently, a poly(vinyl imidazole) electrolytes demonstrated one of the highest conductivities for Ca2+ to date.[33]
Solid electrolytes
Solid electrolytes (i.e., ceramics) have been proposed for the transport of calcium ions, but studies remain theoretical.
Anodes
Calcium anodes have focused on using metal anodes, metal oxides, carbons, as well as metals/semiconductors as alloying compounds.
Examples of anode materials include vanadium oxide (V2O5),[34] copper-calcium alloying,[35] MgV2O5, graphite,[36] metallic calcium,[10] and silicon anodes.[37] Recent work on plating/stripping calcium was done in ethylene carbonate/propylene carbonate (EC/PC) solutions at elevated temperatures.[19] Calcium metal anodes have also shown practical plating at room temperature in different electrolytes such as tetrahydrofuran (THF) and a binary mixture of ethylene carbonate and propylene carbonate (EC/PC).[38][18] Aqueous batteries (namely those employing water as the solvent component of the electrolyte) have used calcium vanadate.[17] Graphene like materials, such as hexa-peri-hexabenzocoronene nanographene, have also been considered as Ca2+ anodes.[39]
Cathodes
Cathode materials for calcium seek to provide suitable material structures for the reliable storage and release of calcium ions. Primary work on calcium cathodes have focused on the experimental and theoretical investigation of intercalation compounds as well as sulfur as a conversion cathode.
Significant progress has been made with employing materials that are generally good intercalation materials for ions, as well as specifically ceramics with crystal structures that provide low migration energy barriers for Ca2+ to move through the lattice. The divalency and large ionic radius of calcium necessitates intercalation hosts with relatively open crystal frameworks and milder crystal polarization to help facilitate better diffusion kinetics. Layered materials, whereby Ca2+ is transported through the van der Waals gap, is also an approach to enable faster diffusion.
Thus far calcium metal oxides and sulfides are areas of study. Cathodes examined recently include calcium manganese oxide,[40] calcium cobalt oxide[34] and titanium disulfide,[41][42] as well as hexacyanoferrates,[43][44] or dual carrier batteries,[45] as well as for aqueous calcium ion batteries.[46] Theoretical work has been performed to ascertain the potential of cathodes from different crystal structures such as perovskite (CaMO3),[47] spinel (CaM2O4),[48][49] other naturally occurring calcium compounds,[50] metal selenides such as TiSe2,[51] as well as other calcium lanthanide oxide phases.
Extensive examinations of the migration energy barriers have also discussed.[4]
Conversion cathodes, such as the use of sulfur, is also a viable solution that may overcome setbacks with intercalation hosts.
Calcium–sulfur batteries
A primary Ca–S battery was examined.[52] Ca-S batteries have also been examined using Li as a mediator to make it reversible.[53] The discovery of reliable electrolytes for plating/stripping calcium metal have aided in stable Ca//S battery cycling, however, poly-sulfide dissolution remains at issue to long term performance.
Calcium–air batteries
In addition, a calcium–air (Ca–O2) batteries have also been examined.[54][55] Unlike Li-O2 batteries, in which lithium can form a superoxide that undergoes easy redox activity, calcium only oxidizes to the extremely chemical stable calcium oxide (CaO), hence suitable catalysts systems are required to aid in the reduction of CaO during battery recharging. Reliable plating and stripping at the Ca anode is also critical to battery performance.
Investigated battery cells and performance metrics
Several calcium metal batteries with different cathodes have thus far been examined: Ca//V2O5,[56] Ca//Ca4Fe9O17,[57] Ca//LiTiO2,[24] Ca/Carbon-Fiber,[25] Ca//TiS2,[42] Ca//FePO4, Ca//Ca3Co2O6, Ca//PAQ, and Ca//S.[58] C-rates range from 0.2 to >5 C. Capacities thus far achieved range from 50-250 mAh/g, with operating voltages between 1 and 4 V. Current densities are in the range of 20–500 mA/g, and energy densities of ~250 Wh/kg.
Applications
Owing in the potentially greater weight of calcium batteries, they have been proposed for use in stationary applications, such as grid storage. Portable electronics as well as electric vehicle applications may be possible if gravimetric capacities and current densities are improved.
Notable research initiatives
There are several groups and consortia dedicated to the aim of producing commercial-grade rechargeable calcium batteries, for example, the CARBAT (Europe) and the Syracuse Center of Excellence (USA), and the Joint Center for Energy Storage Research (USA).
Challenges
Calcium batteries currently show capacity fading and relatively lower energy densities than Li-metal batteries, but there are concerted efforts aiming to overcome these issues.[59] The solid electrolyte interface (SEI) also shows slow migration of Ca2+ ions. Ca metal also undergoes dendritic growth at high current rates.[60] The nature of the calcium deposits are also critical for long-term battery operation, with efforts aiming to produce high quality, uniform deposits. Calcium batteries that provide comparable energy densities of incumbent Li-ion and Li-metal batteries require a pure Ca metal anode to be employed. Calcium is a significantly hard metal compared to lithium, which will have to be addressed for practical integration of calcium foils in battery manufacture, such as pouch and cylindrical cells.
Calcium salts of generally show strong coordination between the Ca2+ and the anion, consequently requiring strongly coordinating solvents, such as carbonates, in order to produce electrolytes with sufficient salt solubility. This results in slow kinetics of plating/stripping at a Ca metal interface. More weakly coordinating salts allow for weakly coordinating solvents to be employed, which shows significantly increase kinetics.[13][14]
Intercalation hosts need to provide open frameworks and simple migration pathways for the transport of calcium ions which is both larger in size (e.g. as compared to Li+) as well as has a greater charge density. This can allow for the materials to enable high charge/discharge rates.
References
- ↑ Hosein ID (2021-04-09). "The Promise of Calcium Batteries: Open Perspectives and Fair Comparisons". ACS Energy Letters. 6 (4): 1560–1565. doi:10.1021/acsenergylett.1c00593.
- ↑ Nielson KV, Liu TL (February 2020). "Dawn of Calcium Batteries". Angewandte Chemie. 59 (9): 3368–3370. doi:10.1002/anie.201913465. PMID 31961466. S2CID 210842839.
- ↑ A Youtube popular science animation explaining Ca batteries - a product of the H2020 project CARBAT (FET-Open), retrieved 2021-06-13
- 1 2 Arroyo-de Dompablo ME, Ponrouch A, Johansson P, Palacín MR (July 2020). "Achievements, Challenges, and Prospects of Calcium Batteries". Chemical Reviews. 120 (14): 6331–6357. doi:10.1021/acs.chemrev.9b00339. hdl:10261/199754. PMID 31661250.
- ↑ Stievano L, de Meatza I, Bitenc J, Cavallo C, Brutti S, Navarra MA (2021-01-15). "Emerging calcium batteries". Journal of Power Sources. 482: 228875. Bibcode:2021JPS...48228875S. doi:10.1016/j.jpowsour.2020.228875. ISSN 0378-7753.
- ↑ Ji B, He H, Yao W, Tang Y (January 2021). "Recent Advances and Perspectives on Calcium-Ion Storage: Key Materials and Devices". Advanced Materials. 33 (2): e2005501. doi:10.1002/adma.202005501. PMID 33251702. S2CID 227237159.
- ↑ Selis SM, Wondowski JP, Justus RF (1964-01-01). "A High‐Rate, High‐Energy Thermal Battery System". Journal of the Electrochemical Society. 111 (1): 6. Bibcode:1964JElS..111....6S. doi:10.1149/1.2426065. ISSN 1945-7111.
- ↑ Staniewicz RJ (1980-04-01). "A Study of the Calcium‐Thionyl Chloride Electrochemical System". Journal of the Electrochemical Society. 127 (4): 782–789. Bibcode:1980JElS..127..782S. doi:10.1149/1.2129758. ISSN 1945-7111.
- ↑ Whittingham MS (1978-01-01). "Chemistry of intercalation compounds: Metal guests in chalcogenide hosts". Progress in Solid State Chemistry. 12 (1): 41–99. doi:10.1016/0079-6786(78)90003-1. ISSN 0079-6786.
- 1 2 Monti D, Ponrouch A, Araujo RB, Barde F, Johansson P, Palacín MR (2019). "Multivalent Batteries-Prospects for High Energy Density: Ca Batteries". Frontiers in Chemistry. 7: 79. Bibcode:2019FrCh....7...79M. doi:10.3389/fchem.2019.00079. PMC 6391315. PMID 30842941.
- ↑ Greenwood NN (1997). Chemistry of the Elements (2nd ed.). Boston, Mass.: Butterworth-Heinemann. ISBN 978-0-7506-3365-9.
- ↑ Muldoon J, Bucur CB, Gregory T (December 2014). "Quest for nonaqueous multivalent secondary batteries: magnesium and beyond". Chemical Reviews. 114 (23): 11683–11720. doi:10.1021/cr500049y. PMID 25343313.
- 1 2 Shyamsunder A, Blanc LE, Assoud A, Nazar LF (2019-09-13). "Reversible Calcium Plating and Stripping at Room Temperature Using a Borate Salt". ACS Energy Letters. 4 (9): 2271–2276. doi:10.1021/acsenergylett.9b01550. ISSN 2380-8195. S2CID 202079165.
- 1 2 Li Z, Fuhr O, Fichtner M, Zhao-Karger Z (2019-12-04). "Towards stable and efficient electrolytes for room-temperature rechargeable calcium batteries". Energy & Environmental Science. 12 (12): 3496–3501. doi:10.1039/C9EE01699F. ISSN 1754-5706.
- ↑ Nielson KV, Luo J, Liu TL (2020). "Optimizing Calcium Electrolytes by Solvent Manipulation for Calcium Batteries". Batteries & Supercaps. 3 (8): 766–772. doi:10.1002/batt.202000005. S2CID 216329867.
- ↑ Aurbach D, Skaletsky R, Gofer Y (1991-12-01). "The Electrochemical Behavior of Calcium Electrodes in a Few Organic Electrolytes". Journal of the Electrochemical Society. 138 (12): 3536–3545. Bibcode:1991JElS..138.3536A. doi:10.1149/1.2085455. ISSN 0013-4651.
- 1 2 Liu L, Wu YC, Rozier P, Taberna PL, Simon P (2019). "Ultrafast Synthesis of Calcium Vanadate for Superior Aqueous Calcium-Ion Battery". Research. 2019: 6585686. Bibcode:2019Resea201985686L. doi:10.34133/2019/6585686. PMC 6944483. PMID 31912041.
- 1 2 Biria S, Pathreeker S, Li H, Hosein ID (2019-11-25). "Plating and Stripping of Calcium in an Alkyl Carbonate Electrolyte at Room Temperature". ACS Applied Energy Materials. 2 (11): 7738–7743. doi:10.1021/acsaem.9b01670. ISSN 2574-0962. S2CID 208759289.
- 1 2 Ponrouch A, Frontera C, Bardé F, Palacín MR (February 2016). "Towards a calcium-based rechargeable battery". Nature Materials. 15 (2): 169–172. Bibcode:2016NatMa..15..169P. doi:10.1038/nmat4462. hdl:10261/148078. PMID 26501412.
- ↑ Shakourian-Fard M, Kamath G, Taimoory SM, Trant JF (2019-07-05). "Calcium-Ion Batteries: Identifying Ideal Electrolytes for Next-Generation Energy Storage Using Computational Analysis". The Journal of Physical Chemistry C. 123 (26): 15885–15896. doi:10.1021/acs.jpcc.9b01655. ISSN 1932-7447. S2CID 197216442.
- ↑ Araujo RB, Thangavel V, Johansson P (2021-08-01). "Towards novel calcium battery electrolytes by efficient computational screening". Energy Storage Materials. 39: 89–95. doi:10.1016/j.ensm.2021.04.015. ISSN 2405-8297. S2CID 234810587.
- ↑ Forero-Saboya JD, Marchante E, Araujo RB, Monti D, Johansson P, Ponrouch A (December 2019). "Cation Solvation and Physicochemical Properties of Ca Battery Electrolytes". The Journal of Physical Chemistry C. 123 (49): 29524–29532. doi:10.1021/acs.jpcc.9b07308. PMC 6961307. PMID 31956392.
- ↑ Biria S, Pathreeker S, Genier FS, Li H, Hosein ID (2020-03-23). "Plating and Stripping Calcium at Room Temperature in an Ionic-Liquid Electrolyte". ACS Applied Energy Materials. 3 (3): 2310–2314. doi:10.1021/acsaem.9b02529. ISSN 2574-0962. S2CID 214030347.
- 1 2 Jie Y, Tan Y, Li L, Han Y, Xu S, Zhao Z, et al. (July 2020). "Electrolyte Solvation Manipulation Enables Unprecedented Room-Temperature Calcium-Metal Batteries". Angewandte Chemie. 59 (31): 12689–12693. doi:10.1002/anie.202002274. PMID 32270534. S2CID 215602284.
- 1 2 Song H, Su J, Wang C (January 2021). "Hybrid Solid Electrolyte Interphases Enabled Ultralong Life Ca-Metal Batteries Working at Room Temperature". Advanced Materials. 33 (2): e2006141. Bibcode:2021AdM....3306141S. doi:10.1002/adma.202006141. PMID 33215793. S2CID 227078025.
- ↑ Vanitha D, Bahadur SA, Nallamuthu N, Shunmuganarayanan A, Manikandan A (March 2018). "Studies on Conducting Polymer Blends: Synthesis and Characterizations of PVA/PVP Doped with CaCl₂". Journal of Nanoscience and Nanotechnology. 18 (3): 1723–1729. doi:10.1166/jnn.2018.14215. PMID 29448651.
- ↑ Genier FS, Burdin CV, Biria S, Hosein ID (2019-02-28). "A novel calcium-ion solid polymer electrolyte based on crosslinked poly(ethylene glycol) diacrylate". Journal of Power Sources. 414: 302–307. Bibcode:2019JPS...414..302G. doi:10.1016/j.jpowsour.2019.01.017. ISSN 0378-7753. S2CID 104435180.
- ↑ Wang J, Genier FS, Li H, Biria S, Hosein ID (2019-07-12). "A Solid Polymer Electrolyte from Cross-Linked Polytetrahydrofuran for Calcium Ion Conduction". ACS Applied Polymer Materials. 1 (7): 1837–1844. doi:10.1021/acsapm.9b00371. ISSN 2637-6105. S2CID 104749306.
- ↑ Martinez-Cisneros CS, Fernandez A, Antonelli C, Levenfeld B, Varez A, Vezzù K, Di Noto V, Sanchez JY (2020-09-01). "Opening the door to liquid-free polymer electrolytes for calcium batteries". Electrochimica Acta. 353: 136525. doi:10.1016/j.electacta.2020.136525. hdl:10016/31257. ISSN 0013-4686. S2CID 219746567.
- ↑ Ford HO, Cui C, Schaefer JL (March 2020). "Comparison of Single-Ion Conducting Polymer Gel Electrolytes for Sodium, Potassium, and Calcium Batteries: Influence of Polymer Chemistry, Cation Identity, Charge Density, and Solvent on Conductivity". Batteries. 6 (1): 11. doi:10.3390/batteries6010011.
- ↑ Biria S, Pathreeker S, Genier FS, Chen FH, Li H, Burdin CV, Hosein ID (July 2021). "Gel Polymer Electrolytes Based on Cross-Linked Poly(ethylene glycol) Diacrylate for Calcium-Ion Conduction". ACS Omega. 6 (26): 17095–17102. doi:10.1021/acsomega.1c02312. PMC 8264931. PMID 34250366.
- ↑ Biria S, Pathreeker S, Genier FS, Hosein ID (2020-06-12). "A Highly Conductive and Thermally Stable Ionic Liquid Gel Electrolyte for Calcium-Ion Batteries". ACS Applied Polymer Materials. 2 (6): 2111–2118. doi:10.1021/acsapm.9b01223. ISSN 2637-6105. S2CID 219098278.
- ↑ Pathreeker S, Hosein ID (October 2022). "Vinylimidazole-Based Polymer Electrolytes with Superior Conductivity and Promising Electrochemical Performance for Calcium Batteries". ACS Applied Polymer Materials. 4 (10): 6803–6811. doi:10.1021/acsapm.2c01140. PMC 9578112. PMID 36277173.
- 1 2 Cabello M, Nacimiento F, Gonzalez JR, Ortiz G, Alcantara R, Lavela P, Perez-Vicente C, Tirado JL (2016-06-01). "Advancing towards a veritable calcium-ion battery: CaCo2O4 positive electrode material". Electrochemistry Communications. 67: 59–64. doi:10.1016/j.elecom.2016.03.016. ISSN 1388-2481.
- ↑ Wang M, Jiang C, Zhang S, Song X, Tang Y, Cheng HM (June 2018). "Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage". Nature Chemistry. 10 (6): 667–672. Bibcode:2018NatCh..10..667W. doi:10.1038/s41557-018-0045-4. PMID 29686378. S2CID 19086248.
- ↑ Richard Prabakar SJ, Ikhe AB, Park WB, Chung KC, Park H, Kim KJ, et al. (December 2019). "Graphite as a Long-Life Ca2+-Intercalation Anode and its Implementation for Rocking-Chair Type Calcium-Ion Batteries". Advanced Science. 6 (24): 1902129. doi:10.1002/advs.201902129. PMC 6918123. PMID 31890464.
- ↑ Ponrouch A, Tchitchekova D, Frontera C, Bardé F, Arroyo-de Dompablo ME, Palacín MR (2016-05-01). "Assessing Si-based anodes for Ca-ion batteries: Electrochemical decalciation of CaSi2". Electrochemistry Communications. 66: 75–78. doi:10.1016/j.elecom.2016.03.004. hdl:10261/147984. ISSN 1388-2481.
- ↑ Wang D, Gao X, Chen Y, Jin L, Kuss C, Bruce PG (January 2018). "Plating and stripping calcium in an organic electrolyte". Nature Materials. 17 (1): 16–20. Bibcode:2018NatMa..17...16W. doi:10.1038/nmat5036. PMID 29180779. S2CID 103355612.
- ↑ Hassanpour A, Farhami N, Derakhshande M, Nezhad PD, Ebadi A, Ebrahimiasl S (2021-07-01). "Magnesium and calcium ion batteries based on the hexa-peri-hexabenzocoronene nanographene anode materials". Inorganic Chemistry Communications. 129: 108656. doi:10.1016/j.inoche.2021.108656. ISSN 1387-7003. S2CID 235542961.
- ↑ Pathreeker S, Reed S, Chando P, Hosein ID (October 2020). "A study of calcium ion intercalation in perovskite calcium manganese oxide". Journal of Electroanalytical Chemistry. 874: 114453. doi:10.1016/j.jelechem.2020.114453. ISSN 1572-6657. S2CID 225409592.
- ↑ Lee C, Jeong YT, Nogales PM, Song HY, Kim Y, Yin RZ, Jeong SK (January 2019). "Electrochemical intercalation of Ca2+ ions into TiS2 in organic electrolytes at room temperature". Electrochemistry Communications. 98: 115–118. doi:10.1016/j.elecom.2018.12.003. ISSN 1388-2481.
- 1 2 Tchitchekova DS, Ponrouch A, Verrelli R, Broux T, Frontera C, Sorrentino A, et al. (February 2018). "Electrochemical Intercalation of Calcium and Magnesium in TiS 2 : Fundamental Studies Related to Multivalent Battery Applications". Chemistry of Materials. 30 (3): 847–856. doi:10.1021/acs.chemmater.7b04406. ISSN 0897-4756.
- ↑ Padigi P, Goncher G, Evans D, Solanki R (January 2015). "Potassium barium hexacyanoferrate – A potential cathode material for rechargeable calcium ion batteries". Journal of Power Sources. 273: 460–464. Bibcode:2015JPS...273..460P. doi:10.1016/j.jpowsour.2014.09.101. ISSN 0378-7753.
- ↑ Tojo T, Sugiura Y, Inada R, Sakurai Y (2016-07-20). "Reversible Calcium Ion Batteries Using a Dehydrated Prussian Blue Analogue Cathode". Electrochimica Acta. 207: 22–27. doi:10.1016/j.electacta.2016.04.159. ISSN 0013-4686.
- ↑ Shiga T, Kondo H, Kato Y, Inoue M (2015-12-17). "Insertion of Calcium Ion into Prussian Blue Analogue in Nonaqueous Solutions and Its Application to a Rechargeable Battery with Dual Carriers". The Journal of Physical Chemistry C. 119 (50): 27946–27953. doi:10.1021/acs.jpcc.5b10245. ISSN 1932-7447.
- ↑ Adil M, Sarkar A, Roy A, Panda MR, Nagendra A, Mitra S (March 2020). "Practical Aqueous Calcium-Ion Battery Full-Cells for Future Stationary Storage". ACS Applied Materials & Interfaces. 12 (10): 11489–11503. doi:10.1021/acsami.9b20129. PMID 32073827. S2CID 211214804.
- ↑ Arroyo-de Dompablo ME, Krich C, Nava-Avendaño J, Palacín MR, Bardé F (July 2016). "In quest of cathode materials for Ca ion batteries: the CaMO3 perovskites (M = Mo, Cr, Mn, Fe, Co, and Ni)". Physical Chemistry Chemical Physics. 18 (29): 19966–19972. Bibcode:2016PCCP...1819966A. doi:10.1039/C6CP03381D. hdl:10261/147901. PMID 27398629.
- ↑ Zhao Z, Yao J, Sun B, Zhong S, Lei X, Xu B, Ouyang C (2018-11-15). "First-principles identification of spinel CaCo2O4 as a promising cathode material for Ca-ion batteries". Solid State Ionics. 326: 145–149. doi:10.1016/j.ssi.2018.10.004. ISSN 0167-2738. S2CID 105010988.
- ↑ Liu D, Zhu W, Trottier J, Gagnon C, Barray F, Guerfi A, et al. (2013-11-18). "Spinel materials for high-voltage cathodes in Li-ion batteries". RSC Advances. 4 (1): 154–167. doi:10.1039/C3RA45706K. ISSN 2046-2069.
- ↑ Torres A, Luque FJ, Tortajada J, Arroyo-de Dompablo ME (July 2019). "Analysis of Minerals as Electrode Materials for Ca-based Rechargeable Batteries". Scientific Reports. 9 (1): 9644. Bibcode:2019NatSR...9.9644T. doi:10.1038/s41598-019-46002-4. PMC 6609692. PMID 31273248.
- ↑ Juran TR, Smeu M (2019-10-01). "TiSe2 cathode for beyond Li-ion batteries". Journal of Power Sources. 436: 226813. Bibcode:2019JPS...43626813J. doi:10.1016/j.jpowsour.2019.226813. ISSN 0378-7753. S2CID 198324987.
- ↑ See KA, Gerbec JA, Jun YS, Wudl F, Stucky GD, Seshadri R (August 2013). "A High Capacity Calcium Primary Cell Based on the Ca-S System". Advanced Energy Materials. 3 (8): 1056–1061. doi:10.1002/aenm.201300160. S2CID 97151846.
- ↑ Yu X, Boyer MJ, Hwang GS, Manthiram A (2019). "Toward a Reversible Calcium-Sulfur Battery with a Lithium-Ion Mediation Approach". Advanced Energy Materials. 9 (14): 1803794. doi:10.1002/aenm.201803794. ISSN 1614-6840. OSTI 1598280.
- ↑ Reinsberg P, Bondue CJ, Baltruschat H (2016-10-06). "Calcium–Oxygen Batteries as a Promising Alternative to Sodium–Oxygen Batteries". The Journal of Physical Chemistry C. 120 (39): 22179–22185. doi:10.1021/acs.jpcc.6b06674. ISSN 1932-7447.
- ↑ Shiga T, Kato Y, Yoko H (2017-06-27). "Coupling of nitroxyl radical as an electrochemical charging catalyst and ionic liquid for calcium plating/stripping toward a rechargeable calcium–oxygen battery". Journal of Materials Chemistry A. 5 (25): 13212–13219. doi:10.1039/C7TA03422A. ISSN 2050-7496.
- ↑ Gao X, Liu X, Mariani A, Elia GA, Lechner M, Streb C, Passerini S (2020-08-13). "Alkoxy-functionalized ionic liquid electrolytes: understanding ionic coordination of calcium ion speciation for the rational design of calcium electrolytes". Energy & Environmental Science. 13 (8): 2559–2569. doi:10.1039/D0EE00831A. ISSN 1754-5706.
- ↑ Black AP, Torres A, Frontera C, Palacín MR, Arroyo-de Dompablo ME (February 2020). "Appraisal of calcium ferrites as cathodes for calcium rechargeable batteries: DFT, synthesis, characterization and electrochemistry of Ca4Fe9O17". Dalton Transactions. 49 (8): 2671–2679. doi:10.1039/C9DT04688G. PMID 32048697.
- ↑ Li Z, Vinayan BP, Diemant T, Behm RJ, Fichtner M, Zhao-Karger Z (October 2020). "Rechargeable Calcium-Sulfur Batteries Enabled by an Efficient Borate-Based Electrolyte". Small. 16 (39): e2001806. doi:10.1002/smll.202001806. PMID 32812367.
- ↑ Palacin M, Black A, Tchitchekova DS, Johansson P, Araujo RB, Aren F, et al. (2020-11-23). "Tackling the Development of Rechargeable Calcium Batteries: The CARBAT Project". ECS Meeting Abstracts. MA2020-02 (2): 449. doi:10.1149/MA2020-022449mtgabs. ISSN 2151-2043. S2CID 234584810.
- ↑ Pu SD, Gong C, Gao X, Ning Z, Yang S, Marie JJ, et al. (2020-07-10). "Current-Density-Dependent Electroplating in Ca Electrolytes: From Globules to Dendrites". ACS Energy Letters. 5 (7): 2283–2290. doi:10.1021/acsenergylett.0c01153. S2CID 225648185.