| Capitella teleta | |
|---|---|
 | |
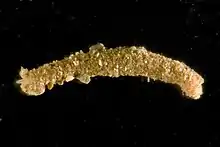
| Adult male | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Annelida |
| Clade: | Pleistoannelida |
| Subclass: | Sedentaria |
| Family: | Capitellidae |
| Genus: | Capitella |
| Species: | C. teleta |
| Binomial name | |
| Capitella teleta Blake, Grassle & Eckelbarger, 2009 | |
Capitella teleta is a small, cosmopolitan, segmented annelid worm. It is a well-studied invertebrate, which has been cultured for use in laboratories for over 30 years.[1] C. teleta is the first marine polychaete to have its genome sequenced.[2][3]
Description
Initial discovery
For many years researchers believed that Capitella capitata was the only representative of this genus that survived, and flourished, in polluted environments. After the oil spill that occurred near Cape Cod in West Falmouth, Massachusetts in 1969, researchers collected sediment and found an abundance of what they believed to be C. capitata.[4][5] However, subsequent research showed that while the individuals collected from that region had very similar gross morphology, their life histories, methods of reproduction and genetics indicated there were at least six distinct species. Capitella species I, eventually described as Capitella teleta in 2009, was one of the initial species identified from these surveys.[6]
Etymology
After 30 years of research on the group, Capitella teleta was officially described in 2009 by Blake et al. The species name is derived from the Greek word teleta, meaning "initiation". This word symbolizes that it was the first alternative Capitella species that was identified.[4]
Phylogenetics
A 2018 molecular phylogeny of the family Capitellidae established clear monophyly and showed 8 genera.[7] The phylogeny utilized 36 capitellid species and combined data from 18S, 28S, H3, and COI gene sequences. This study also established Capitellidae as the sister group to Echiura. While the study attempted to map morphological characters to the molecular phylogeny, this was not phylogenetically informative and a more detailed re-evaluation of morphology could help to elucidate character trait evolution.
Taxonomic morphology
Capitella teleta has a narrow, segmented body with reduced parapodia and is red in color. There are nine anterior thoracic segments and many more abdominal segments. New segments are added throughout the lifespan from a posterior subterminal growth zone called the posterior growth zone. Like other polychaetes, C. teleta has fine bristles or setae. Setae are segmentally repeated along the body, with morphologically distinct setae in the thoracic (hooded hooks) and abdominal segments (capillary setae).[4] This animal exhibits sexual dimorphism and males have dorsally-positioned genital spines on setigers 7–8 while females have paired ovaries in the abdominal segments.[8] Generally, there are separate sexes; however, hermaphroditism is possible when there are low densities of females. Males, females and hermaphrodites are of similar size (maximum size collected was a male that is 24 mm in length).[4][9]
Ecology
Habitat
Capitella teleta lives in the shallow-water or intertidal marine environment. It is also found in salt marshes and is often found in high concentrations in disturbed soft sediments. It is a member of the infaunal benthic community. C. teleta burrows through the sediment by peristalsis, using its hydrostatic skeleton and contraction of longitudinal and circular muscles in the body wall. The thoracic segments of C. teleta also contain helical muscles that are proposed to generate additional force for burrowing.[10] Capitellids are commonly thought of as opportunistic in nature, due to their ability to inhabit and flourish in organically enriched marine sediments.[4][5]
This organism is commonly found in sediments along the east and west coasts of North America. Additional reports have placed this group in the Mediterranean region as well as Japan.[4][9]
Life history
Capitella teleta embryos and early larval stages develop in a brood tube that surrounds the mother.[11] The embryos are approximately 200 µm in diameter.[6] Over the course of approximately a week, the embryos develop into non-feeding larvae which form musculature, a centralized nervous system, two circular ciliary bands, two eye spots, segments, and setae. The larvae are non-feeding and the digestive system develops at a later stage than other organs. Pre-metamorphosis larvae can be categorized into nine stages, with each stage lasting approximately one day.[12] Upon further body elongation and gut maturation, the larvae emerge from the brood tube, and swim forward with a rotational turn via the beating of cilia organized within two circular bands, the prototroch and telotroch.[11] Larvae exhibit positive phototactic behavior in which they swim towards light, potentially an adaptation to aid in larval dispersal[13][14][15] C. teleta is an indirect developer and undergoes metamorphosis from a swimming larva into a burrowing juvenile. Metamorphosis is characterized by cilia loss, body elongation, and crawling behavior.[16] Marine sediment functions as a cue to initiate metamorphosis into juvenile worms that thereafter grow into mature adults.[15] Competent larvae can be induced to metamorphose into juveniles when exposed to the B vitamins Nicotinamide (B3) and Riboflavin (B2), suggesting that these chemical compounds may be responsible for the inductive role of the marine sediment in larval metamorphosis.[17] The number of offspring in each brood tube can vary between 50 - 400 individuals,[6] and is influenced by food quality.[18]
After metamorphosis, the juveniles begin burrowing and feeding. The juvenile worms continue to grow and add segments during the eight weeks it takes to become sexually mature adults. Males and females can reproduce multiple times during their lifetime. Adults live approximately 12–14 weeks after maturation.

Feeding
Capitella teleta feeds on the enriched sediment in which it burrows. C. teleta has a complex, regionalized alimentary canal consisting of a foregut, midgut and hindgut.[19] It ingests the sediment by everting its proboscis, which contains a ciliated, muscular dorsal pharynx.[20] Presence of a dorsal pharynx is uncommon in marine polychaetes, and this adaptation may have evolved independently in the family Capitellidae through selective pressures on feeding mode in the benthic marine niche they occupy.[20]
Research

A wide range of techniques have been developed to investigate C. teleta developmental processes. In 2006, the first study using whole mount in situ hybridization was published.[20][21] This technique allows investigation of the expression and localization of specific mRNAs within a fixed sample. Immunohistochemistry was later developed as a way to visualize specific cell types in fixed specimens.[22] A microinjection protocol for uncleaved embryos and early cleavage stages was developed in 2010 and was used in a fate mapping study[23] to investigate the ultimate fate of blastomeres.[24][25] Other useful techniques for studying early development of the embryo are targeted deletion of single cells with an infrared laser and blastomere isolation experiments.[11][26][13][27][28] Laser deletion was also utilized for the deletion of larval eyes at a later stage in development.[15] The development of microinjection techniques allowed for introduction of different nucleic acid constructs that can be injected into an uncleaved zygote. This includes use of gene perturbation techniques such as Morpholino knockdown and CRISPR-Cas9 mutagenesis, and methods for living imaging such as mRNA injection.[29][30][31] The development of each technique opens doors for new avenues of inquiry and experimentation and expands the number and complexity of questions C. teleta researchers can thoroughly investigate.
Embryogenesis
Like many species within Spiralia, C. teleta embryogenesis follows an unequal spiral cleavage program where blastomeres are born according to a predictable order, size and position. This shared stereotypic cleavage program allows for the identification of individual cells and there is a standard cell-nomenclature system. Additionally, individual cells can be microinjected with fluorescent dyes and their descendants tracked to determine the lineage of particular tissues and larval structures. Through this method, a comprehensive fate map was created for C. teleta.[23] In general, there is substantial similarity of cell fates between C. teleta and other Spiralia.[32][23][31] For instance, in C. teleta and several other spiralians, cells derived from the A, B, C, and D embryo quadrants respectively give rise to the left, ventral, right, and dorsal portions of the larval body.[23] However, the origin of mesoderm differs across species. In C. teleta, mesoderm is generated from cells called 3c and 3d that are derived from both the C and D embryo quadrants, but in the annelid Platynereis dumerilii and in several mollusks, trunk mesoderm is generated from a single cell 4d.[33][34][35][36][37]
The establishment of the dorsal-ventral axis during early embryological development has also been extensively studied in C. teleta. It is reported that micromere 2d, a cell that is born when the embryo has 16 cells, has organizing activity which enables it to induce dorsal-ventral polarity within the embryo.[13] Fate map studies have demonstrated that cell 2d gives rise to ectoderm in the larval trunk and pygidium in C. teleta,[24] while descendants of the first quartet micromeres give rise to structures in the larval head. When micromere 2d is laser ablated, 2d derived structures as well as dorsal-ventral organization in the head is lost.[13] This suggests a requirement for 2d to be present in order to induce the proper formation of the head along a dorsal-ventral axis. When micromeres 2d1 and 2d2, the immediate descendants of 2d, are both deleted, the resulting larvae retain dorsal-ventral organization within the head.[13] It was therefore concluded that in C. teleta micromere 2d has organizing activity in patterning the dorsal-ventral body axis. Furthermore, perturbation studies have shown that the dorsal-ventral axis is primarily patterned via the Activin/Nodal pathway.[38]
Regeneration
Many annelids possess the capability to regenerate their anterior, posterior, or both ends of their body.[39] C. teleta is capable of posterior regeneration.[40][41][42] Both juveniles and adults can regenerate their posterior halves quite well. A staging system has been established, describing the sequential regeneration events in juveniles of C. teleta.[43] The first stage of regeneration encompasses the first 24 hours following amputation or injury. This stage is marked by wound healing and a change in cell proliferation patterns. Wound healing occurs within 4–6 hours of amputation, as the circular muscles in the body wall contract, bringing the epithelium together to cover the wound. During this time, cell proliferation patterns are different from uncut animals; while cell proliferation is still observed throughout the body, there is a marked reduction at the wound site. In stage II, approximately 2 days after amputation, a small blastema forms that contains proliferating cells, and there is a diffuse network of neurites extending from the old ventral nerve cord tissue into the blastema. In stage III, approximately 3 days after amputation, the blastema becomes more organized as proliferating cells pack closely together in the newly formed tissue and multiple neurites condense into nerves. In stage IV, 5 days after amputation, there continues to be an increase in cell proliferation, but less so in the new tissue. The neural projections into the blastema become even more organized and patterned. Additionally, the posterior growth zone, pygidium, and hindgut reform. Finally, Stage 5 is marked by the presence and continued addition of new segments with differentiated tissues and ganglia.[44][45] The entire regeneration process in C. teleta adults is completed within about two weeks[46] The rate of regeneration can vary among individuals, especially pertaining to health and nutrition intake.
Hox genes, patterning genes that regulate segment identity during development in many animals, and are expressed in the blastema of C. teleta during posterior regeneration. This suggests a role in the regeneration process, but the exact expression patterns do not make an obvious link to establishment of segment identity in newly formed tissue during regeneration.[42] The shift in Hox gene expression in the blastema during posterior regeneration is indicative of limited morphallaxis, in addition to epimorphic regeneration[42]
The regeneration of the germline in embryos has also been investigated. In early stage embryos, the germline precursor (cell 3D) was deleted using an infrared laser. 13% of screened larvae showed presence of multipotent progenitor cells (MPCs), indicating some regeneration of the germline. Furthermore, all juveniles two weeks post-metamorphosis have MPCs. Finally, almost all adult worms raised from treated embryos developed functional reproductive systems and produced offspring that developed into swimming larvae.[28]
Toxicology
Capitella teleta is an indicator species for environments contaminated with organic pollution. C. teleta has the ability to colonize these habitats rapidly with high growth rates.[4][47] These characteristics have led to their use in various toxicological studies. Their population and/or individual- level responses to pollutant exposures have been investigated in various toxicants such as synthetic musk,[48] acetyl cedrene,[49] fluoranthene,[50] benzo[a]pyrene,[51] fluoxetine,[52] cadmium,[53] copper oxide nanoparticles,[54] and silver nanoparticles.[52] Recently, the effects of the fluoranthene-spiked sediments on the gut microbiome were investigated and several taxa of bacteria were identified; these taxa may play a role in the metabolism of fluoranthene.[55]
Genome
The genome of Capitella teleta was sequenced in concert with the owl limpet, Lottia gigantean, and the freshwater leech, Helobdella robusta, by the Joint Genome Institute in 2013.[2][3] This was the first attempt at sequencing a marine polychaete and the sequencing and study of these three spiralian genomes provided an important perspective of early bilaterian evolutionary processes. Additionally, this work showed strong support for the monophyletic grouping of Lophotrochozoa.
The researchers found that when compared to other animal genomes, all three organisms possessed genome organization, gene structure and functional content that was more closely related to invertebrate deuterostome genomes than those of fellow invertebrate protostomes. C. teleta possesses a highly conserved and slowly evolving genome with respect to other metazoans.[3][56]
Karyotype analysis revealed that C. teleta has 10 pairs of chromosomes.[57]
References
- ↑ Blake, James A.; Grassle, Judith P.; Eckelbarger, Kevin J. (2009). "Capitella teleta, a new species designation for the opportunistic and experimental Capitella sp. I, with a review of the literature for confirmed records". Zoosymposia. 2: 25–53. doi:10.11646/zoosymposia.2.1.6.
- 1 2 "Home - Capitella sp. I ESC-2004". genome.jgi.doe.gov. Retrieved 2017-04-21.
- 1 2 3 Simakov, Oleg; Marletaz, Ferdinand; Cho, Sung-Jin; Edsinger-Gonzales, Eric; Havlak, Paul; Hellsten, Uffe; Kuo, Dian-Han; Larsson, Tomas; Lv, Jie (2013-01-24). "Insights into bilaterian evolution from three spiralian genomes". Nature. 493 (7433): 526–531. Bibcode:2013Natur.493..526S. doi:10.1038/nature11696. ISSN 0028-0836. PMC 4085046. PMID 23254933.
- 1 2 3 4 5 6 7 Grassle, JF; Grassle, JP (1974). "Opportunistic life histories and genetic systems in marine benthic polychaetes". Journal of Marine Research. 32: 253–284.
- 1 2 L., Sanders, Howard; Frederick, Grassle, J.; R., Hampson, George; S., Morse, Linda; Susan, Garner-Price; C., Jones, Carol (1980-05-01). "Anatomy of an oil spill : long-term effects from the grounding of the barge Florida off West Falmouth, Massachusetts". hdl:1912/3474.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - 1 2 3 Grassle, Judith P.; Grassle, J. Frederick (1976-01-01). "Sibling Species in the Marine Pollution Indicator Capitella (Polychaeta)". Science. 192 (4239): 567–569. Bibcode:1976Sci...192..567G. doi:10.1126/science.1257794. JSTOR 1741571. PMID 1257794.
- ↑ Tomioka, Shinri; Kakui, Keiichi; Kajihara, Hiroshi (October 2018). "Molecular Phylogeny of the Family Capitellidae (Annelida)". Zoological Science. 35 (5): 436–445. doi:10.2108/zs180009. hdl:2115/75605. ISSN 0289-0003. PMID 30298787. S2CID 52936754.
- ↑ Eckelbarger KJ, Grassle JP: Ultrastructural differences in the eggs and ovarian follicle cells of the Capitella (Polychaeta) sibling species. Biol Bull 1983, 165:379-393.
- 1 2 Tomioka, Shinri; Kondoh, Tomohiko; Sato-Okoshi, Waka; Ito, Katsutoshi; Kakui, Keiichi; Kajihara, Hiroshi (2016-10-01). "Cosmopolitan or Cryptic Species? A Case Study of Capitella teleta (Annelida: Capitellidae)". Zoological Science. 33 (5): 545–554. doi:10.2108/zs160059. hdl:2115/68333. ISSN 0289-0003. PMID 27715419. S2CID 25325519.
- ↑ Grill, S.; Dorgan, K. M. (2015-05-15). "Burrowing by small polychaetes - mechanics, behavior and muscle structure of Capitella sp". Journal of Experimental Biology. 218 (10): 1527–1537. doi:10.1242/jeb.113183. ISSN 0022-0949. PMID 25827841.
- 1 2 3 Pernet, Bruno; Amiel, Aldine; Seaver, Elaine C. (2012). "Effects of maternal investment on larvae and juveniles of the annelid Capitella teleta determined by experimental reduction of embryo energy content". Invertebrate Biology. 131 (2): 82–95. doi:10.1111/j.1744-7410.2012.00263.x. ISSN 1744-7410.
- ↑ Seaver, Elaine C.; Thamm, Katrin; Hill, Susan D. (July 2005). "Growth patterns during segmentation in the two polychaete annelids, Capitella sp. I and Hydroides elegans: comparisons at distinct life history stages". Evolution & Development. 7 (4): 312–326. doi:10.1111/j.1525-142X.2005.05037.x. ISSN 1520-541X. PMID 15982368. S2CID 22634415.
- 1 2 3 4 5 Amiel, Aldine R.; Henry, Jonathan Q.; Seaver, Elaine C. (July 2013). "An organizing activity is required for head patterning and cell fate specification in the polychaete annelid Capitella teleta: New insights into cell–cell signaling in Lophotrochozoa". Developmental Biology. 379 (1): 107–122. doi:10.1016/j.ydbio.2013.04.011. PMID 23608454.
- ↑ Butman, CA; Grassle, JP; Buskey, EJ (1988). "Horizontal swimming and gravitational sinking of Capitella sp. I(Annelida: Polychaeta) larvae: implications for settlement". Ophelia. 29: 43–57. doi:10.1080/00785326.1988.10430818.
- 1 2 3 Yamaguchi, Emi; Seaver, Elaine C. (December 2013). "The importance of larval eyes in the polychaete Capitella teleta : effects of larval eye deletion on formation of the adult eye". Invertebrate Biology. 132 (4): 352–367. doi:10.1111/ivb.12034.
- ↑ Biggers, William J.; Pires, Anthony; Pechenik, Jan A.; Johns, Eric; Patel, Priyam; Polson, Theresa; Polson, John (March 2012). "Inhibitors of nitric oxide synthase induce larval settlement and metamorphosis of the polychaete annelid Capitella teleta". Invertebrate Reproduction & Development. 56 (1): 1–13. doi:10.1080/07924259.2011.588006. ISSN 0792-4259. S2CID 38343762.
- ↑ Burns, Robert T.; Pechenik, Jan A.; Biggers, William J.; Scavo, Gia; Lehman, Christopher (2014-11-12). Harder, Tilmann (ed.). "The B Vitamins Nicotinamide (B3) and Riboflavin (B2) Stimulate Metamorphosis in Larvae of the Deposit-Feeding Polychaete Capitella teleta: Implications for a Sensory Ligand-Gated Ion Channel". PLOS ONE. 9 (11): e109535. Bibcode:2014PLoSO...9j9535B. doi:10.1371/journal.pone.0109535. ISSN 1932-6203. PMC 4229104. PMID 25390040.
- ↑ Marsh, A. G.; Gémare, A.; Tenore, K. R. (1989-09-01). "Effect of food type and ration on growth of juvenile Capitella sp. I (Annelida: Polychaeta): macro- and micronutrients". Marine Biology. 102 (4): 519–527. doi:10.1007/BF00438354. ISSN 1432-1793. S2CID 84340221.
- ↑ Boyle, Michael J.; Seaver, Elaine C. (2008-01-03). "Developmental expression of foxA and gata genes during gut formation in the polychaete annelid, Capitella sp. I: Gut development in Capitella sp. I". Evolution & Development. 10 (1): 89–105. doi:10.1111/j.1525-142X.2007.00216.x. PMID 18184360. S2CID 39230534.
- 1 2 3 Boyle, Michael J.; Seaver, Elaine C. (2009-08-31). "Evidence of a dorsal pharynx in the marine polychaete Capitella teleta (Polychaeta: Capitellidae)". Zoosymposia. 2 (1): 317–328. doi:10.11646/zoosymposia.2.1.22. ISSN 1178-9913.
- ↑ Seaver, Elaine C.; Kaneshige, Lori M. (2006-01-01). "Expression of 'segmentation' genes during larval and juvenile development in the polychaetes Capitella sp. I and H. elegans". Developmental Biology. 289 (1): 179–194. doi:10.1016/j.ydbio.2005.10.025. ISSN 0012-1606. PMID 16330020.
- ↑ Meyer, Néva P; Carrillo-Baltodano, Allan; Moore, Richard E; Seaver, Elaine C (December 2015). "Nervous system development in lecithotrophic larval and juvenile stages of the annelid Capitella teleta". Frontiers in Zoology. 12 (1): 15. doi:10.1186/s12983-015-0108-y. ISSN 1742-9994. PMC 4498530. PMID 26167198.
- 1 2 3 4 Meyer, Néva P.; Boyle, Michael J.; Martindale, Mark Q.; Seaver, Elaine C. (2010-09-15). "A comprehensive fate map by intracellular injection of identified blastomeres in the marine polychaete Capitella teleta". EvoDevo. 1 (1): 8. doi:10.1186/2041-9139-1-8. ISSN 2041-9139. PMC 2949861. PMID 20849573.
- 1 2 Meyer, Néva P.; Seaver, Elaine C. (2010-11-01). "Cell Lineage and Fate Map of the Primary Somatoblast of the Polychaete Annelid Capitella teleta". Integrative and Comparative Biology. 50 (5): 756–767. doi:10.1093/icb/icq120. ISSN 1540-7063. PMID 21558238.
- ↑ Meyer, Néva P.; Seaver, Elaine C. (November 2009). "Neurogenesis in an annelid: Characterization of brain neural precursors in the polychaete Capitella sp. I". Developmental Biology. 335 (1): 237–252. doi:10.1016/j.ydbio.2009.06.017. PMID 19540831.
- ↑ Carrillo-Baltodano, Allan M.; Meyer, Néva P. (2017-11-15). "Decoupling brain from nerve cord development in the annelid Capitella teleta: Insights into the evolution of nervous systems". Developmental Biology. 431 (2): 134–144. doi:10.1016/j.ydbio.2017.09.022. ISSN 0012-1606. PMID 28943340.
- ↑ Yamaguchi, Emi; Dannenberg, Leah C.; Amiel, Aldine R.; Seaver, Elaine C. (February 2016). "Regulative capacity for eye formation by first quartet micromeres of the polychaete Capitella teleta". Developmental Biology. 410 (1): 119–130. doi:10.1016/j.ydbio.2015.12.009. PMID 26702513.
- 1 2 Dannenberg, Leah C.; Seaver, Elaine C. (August 2018). "Regeneration of the germline in the annelid Capitella teleta". Developmental Biology. 440 (2): 74–87. doi:10.1016/j.ydbio.2018.05.004. PMID 29758179.
- ↑ Klann, Marleen; Seaver, Elaine C. (December 2019). "Functional role of pax6 during eye and nervous system development in the annelid Capitella teleta". Developmental Biology. 456 (1): 86–103. doi:10.1016/j.ydbio.2019.08.011. PMID 31445008.
- ↑ Neal, S.; de Jong, D. M.; Seaver, E. C. (2019-06-12). "CRISPR/CAS9 mutagenesis of a single r-opsin gene blocks phototaxis in a marine larva". Proceedings of the Royal Society B: Biological Sciences. 286 (1904): 20182491. doi:10.1098/rspb.2018.2491. ISSN 0962-8452. PMC 6571462. PMID 31161907.
- 1 2 Seaver, Elaine C (2016-08-01). "Annelid models I: Capitella teleta". Current Opinion in Genetics & Development. Developmental mechanisms, patterning and evolution. 39: 35–41. doi:10.1016/j.gde.2016.05.025. ISSN 0959-437X. PMID 27318692. S2CID 6151542.
- ↑ Lambert, J. David (2008). "Mesoderm in spiralians: the organizer and the 4d cell". Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 310B (1): 15–23. doi:10.1002/jez.b.21176. ISSN 1552-5015. PMID 17577229.
- ↑ Ackermann, Christian; Dorresteijn, Adriaan; Fischer, Albrecht (December 2005). "Clonal domains in postlarval Platynereis dumerilii (Annelida: Polychaeta)". Journal of Morphology. 266 (3): 258–280. doi:10.1002/jmor.10375. ISSN 0362-2525. PMID 16170805. S2CID 41134309.
- ↑ Conklin, Edwin Grant (1897). "The embryology of crepidula, A contribution to the cell lineage and early development of some marine gasteropods". Journal of Morphology. 13 (1): 1–226. doi:10.1002/jmor.1050130102. hdl:1912/605. ISSN 1097-4687. S2CID 86326127.
- ↑ Dictus, Wim J. A. G; Damen, Peter (1997-03-01). "Cell-lineage and clonal-contribution map of the trochophore larva of Patella vulgata (Mollusca)1Both authors contributed equally to this work.1". Mechanisms of Development. 62 (2): 213–226. doi:10.1016/S0925-4773(97)00666-7. ISSN 0925-4773. PMID 9152012.
- ↑ Lambert, J. David; Johnson, Adam B.; Hudson, Chelsea N.; Chan, Amanda (2016-08-08). "Dpp/BMP2-4 Mediates Signaling from the D-Quadrant Organizer in a Spiralian Embryo". Current Biology. 26 (15): 2003–2010. doi:10.1016/j.cub.2016.05.059. ISSN 0960-9822. PMID 27397892.
- ↑ Lambert, J. David; Nagy, Lisa M. (2001). "MAPK signaling by the D quadrant embryonic organizer of the mollusc Ilyanassa obsoleta". Development. 128 (1): 45–56. doi:10.1242/dev.128.1.45. PMID 11092810.
- ↑ Lanza, Alexis R.; Seaver, Elaine C. (2018-03-01). "An organizing role for the TGF-β signaling pathway in axes formation of the annelid Capitella teleta". Developmental Biology. 435 (1): 26–40. doi:10.1016/j.ydbio.2018.01.004. ISSN 0012-1606. PMID 29337130.
- ↑ Bely, Alexandra E. (2006-08-01). "Distribution of segment regeneration ability in the Annelida". Integrative and Comparative Biology. 46 (4): 508–518. doi:10.1093/icb/icj051. ISSN 1540-7063. PMID 21672762.
- ↑ Hill, Susan D.; Savage, Robert M. (2009-01-01). Shain, Daniel H. (ed.). Annelids in Modern Biology. John Wiley & Sons, Inc. pp. 88–115. doi:10.1002/9780470455203.ch6. ISBN 9780470455203.
- ↑ Giani, Vincent C.; Yamaguchi, Emi; Boyle, Michael J.; Seaver, Elaine C. (2011-05-05). "Somatic and germline expression of piwi during development and regeneration in the marine polychaete annelid Capitella teleta". EvoDevo. 2: 10. doi:10.1186/2041-9139-2-10. ISSN 2041-9139. PMC 3113731. PMID 21545709.
- 1 2 3 Jong, Danielle M. de; Seaver, Elaine C. (2016-02-19). "A Stable Thoracic Hox Code and Epimorphosis Characterize Posterior Regeneration in Capitella teleta". PLOS ONE. 11 (2): e0149724. Bibcode:2016PLoSO..1149724D. doi:10.1371/journal.pone.0149724. ISSN 1932-6203. PMC 4764619. PMID 26894631.
- ↑ name=":22">Jong, Danielle M. de; Seaver, Elaine C. (2016-02-19). "A Stable Thoracic Hox Code and Epimorphosis Characterize Posterior Regeneration in Capitella teleta". PLOS ONE. 11 (2): e0149724. Bibcode:2016PLoSO..1149724D. doi:10.1371/journal.pone.0149724. ISSN 1932-6203. PMC 4764619. PMID 26894631.
- ↑ de Jong, Danielle M.; Seaver, Elaine C. (2016-02-19). Schubert, Michael (ed.). "A Stable Thoracic Hox Code and Epimorphosis Characterize Posterior Regeneration in Capitella teleta". PLOS ONE. 11 (2): e0149724. Bibcode:2016PLoSO..1149724D. doi:10.1371/journal.pone.0149724. ISSN 1932-6203. PMC 4764619. PMID 26894631.
- ↑ de Jong, Danielle M.; Seaver, Elaine C. (March 2018). "Investigation into the cellular origins of posterior regeneration in the annelid Capitella teleta". Regeneration. 5 (1): 61–77. doi:10.1002/reg2.94. PMC 5911572. PMID 29721327.
- ↑ Bely, Alexandra E. (2006-08-01). "Distribution of segment regeneration ability in the Annelida". Integrative and Comparative Biology. 46 (4): 508–518. doi:10.1093/icb/icj051. ISSN 1540-7063. PMID 21672762.
- ↑ Grassle, Judith (1980), "Polychaete Sibling Species", in Brinkhurst, Ralph O.; Cook, David G. (eds.), Aquatic Oligochaete Biology, Springer US, pp. 25–32, doi:10.1007/978-1-4613-3048-6_3, ISBN 978-1-4613-3050-9
- ↑ Ramskov, Tina; Selck, Henriette; Salvitod, Daniel; Forbes, Valery E. (2009). "Individual- and population-level effects of the synthetic musk, hhcb, on the deposit-feeding polychaete, Capitella sp. I". Environmental Toxicology and Chemistry. 28 (12): 2695–2705. doi:10.1897/08-522.1. ISSN 1552-8618. PMID 19788341. S2CID 31694252.
- ↑ Dai, Lina; Selck, Henriette; Salvito, Daniel; Forbes, Valery E. (2012). "Fate and effects of acetyl cedrene in sediments inhabited by different densities of the deposit feeder, Capitella teleta". Environmental Toxicology and Chemistry. 31 (11): 2639–2646. doi:10.1002/etc.1991. ISSN 1552-8618. PMID 22912158. S2CID 25319882.
- ↑ Bach, Lis; Palmqvist, Annemette; Rasmussen, Lene Juel; Forbes, Valery E. (2005-09-30). "Differences in PAH tolerance between Capitella species: Underlying biochemical mechanisms". Aquatic Toxicology. 74 (4): 307–319. doi:10.1016/j.aquatox.2005.06.002. ISSN 0166-445X. PMID 16023227.
- ↑ Palmqvist, Annemette; Rasmussen, Lene Juel; Forbes, Valery E. (2008). "Relative impact of coexposure compared to single-substance exposure on the biotransformation and toxicity of benzo[a]pyrene and fluoranthene in the marine polychaete Capitella sp. I". Environmental Toxicology and Chemistry. 27 (2): 375–386. doi:10.1897/07-156R.1. ISSN 1552-8618. PMID 18348624. S2CID 207267009.
- 1 2 Méndez, Nuria; Lacorte, Silvia; Barata, Carlos (2013-08-01). "Effects of the pharmaceutical fluoxetine in spiked-sediments on feeding activity and growth of the polychaete Capitella teleta". Marine Environmental Research. 89: 76–82. doi:10.1016/j.marenvres.2013.05.004. ISSN 0141-1136. PMID 23769338.
- ↑ Selck, Henriette; Decho, Alan W.; Forbes, Valery E. (1999). "Effects of chronic metal exposure and sediment organic matter on digestive absorption efficiency of cadmium by the deposit-feeding polychaete Capitella species I". Environmental Toxicology and Chemistry. 18 (6): 1289–1297. doi:10.1002/etc.5620180631. ISSN 1552-8618. S2CID 247666449.
- ↑ Dai, Lina; Banta, Gary T.; Selck, Henriette; Forbes, Valery E. (2015-10-01). "Influence of copper oxide nanoparticle form and shape on toxicity and bioaccumulation in the deposit feeder, Capitella teleta". Marine Environmental Research. Particles in the Oceans: Implication for a safe marine environment. 111: 99–106. doi:10.1016/j.marenvres.2015.06.010. ISSN 0141-1136. PMID 26138270.
- ↑ Hochstein, Rebecca; Zhang, Qian; Sadowsky, Michael J.; Forbes, Valery E. (June 2019). "The deposit feeder Capitella teleta has a unique and relatively complex microbiome likely supporting its ability to degrade pollutants". Science of the Total Environment. 670: 547–554. Bibcode:2019ScTEn.670..547H. doi:10.1016/j.scitotenv.2019.03.255. PMID 30909032. S2CID 85514701.
- ↑ Seaver, Elaine C (2016). "Annelid models I: Capitella teleta". Current Opinion in Genetics & Development. 39: 35–41. doi:10.1016/j.gde.2016.05.025. PMID 27318692. S2CID 6151542.
- ↑ GRASSLE, J. P; GELFMAN, C. E.; MILLS, S. W. (1987). "Karyotypes of Capitella Sibling Species, and a Several Species in the Related Genera Capitellides and Capitomastus (Polychaeta)". Bulletin of the Biological Society of Washington (7): 77–88. ISSN 0097-0298.
External links
- Lab Website of Elaine Seaver
- Capitella research YouTube Channel
- Genome page for Capitella teleta: