 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2H-1,3,2-Benzodioxaborole | |
| Other names
7,9-dioxa-8λ2-borabicyclo[4.3.0]nona-1,3,5-triene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.005.447 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H5BO2 | |
| Molar mass | 119.92 g/mol |
| Appearance | Colorless liquid |
| Density | 1.125 g/cm3, liquid |
| Melting point | 12 °C (54 °F; 285 K) |
| Boiling point | 50 °C (122 °F; 323 K) at 50 mmHg |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H225, H314 | |
| P210, P233, P240, P241, P242, P243, P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P370+P378, P403+P235, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 2 °C (36 °F; 275 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
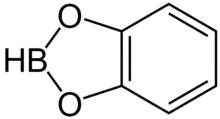
Catecholborane (abbreviated HBcat) is an organoboron compound that is useful in organic synthesis. This colourless liquid is a derivative of catechol and a borane, having the formula C6H4O2BH.
Synthesis and structure
Traditionally catecholborane is produced by treating catechol with borane (BH3) in a cooled solution of THF. However, this method results in a loss of 2 mole equivalents of the hydride. Nöth and Männig described the reaction of alkali-metal boron hydride (LiBH4, NaBH4, of KBH4) with tris(catecholato)bisborane in an ethereal solvent such as diethyl ether.[1] In 2001, Herbert Brown and coworkers prepared catecholborane by treatment of tri-o-phenylene bis-borate with diborane.[2]
Unlike borane itself or alkylboranes, catechol borane exists as a monomer. This behavior is a consequence of the electronic influence of the aryloxy groups that diminish the Lewis acidity of the boron centre. Pinacolborane adopts a similar structure.
Reactions
Catecholborane is less reactive in hydroborations than borane-THF or borane-dimethylsulfide.
When catecholborane is treated with a terminal alkyne, a trans vinylborane is formed:
- C6H4O2BH + HC2R → C6H4O2B-CHCHR
The product is a precursor to the Suzuki reaction and is the only borane which stops at the alkene instead of reacting further to the alkane. [3][4]
Catecholborane may be used as a stereoselective reducing agent when converting β-hydroxy ketones to syn 1,3-diols.
Catecholborane oxidatively adds to low valent metal complexes, affording boryl complexes.[5]
- C6H4O2BH + Pt(PR3)2 → (C6H4O2B)Pt(PR3)2H
References
- ↑ Process for producing catecholborane – Patent 4739096
- ↑ Kanth, Josyula V. B.; Periasamy, Mariappan; Brown, Herbert C. (2000). "New Economical, Convenient Procedures for the Synthesis of Catecholborane". Organic Process Research & Development. 4 (6): 550–553. doi:10.1021/op000291w.
- ↑ Janice Gorzynski Smith, Organic Chemistry: Second Ed. 2008. pp 1007
- ↑ Miyaura, Norio; Suzuki, Akira (1990). "Palladium-Catalyzed Reaction of 1-Alkenylboronates with Vinylic Halides: (1Z,3E)-1-Phenyl-1,3-octadiene". Organic Syntheses. 68: 130. doi:10.15227/orgsyn.068.0130.
- ↑ Neeve, Emily C.; Geier, Stephen J.; Mkhalid, Ibraheem A. I.; Westcott, Stephen A.; Marder, Todd B. (2016). "Diboron(4) Compounds: From Structural Curiosity to Synthetic Workhorse". Chemical Reviews. 116 (16): 9091–9161. doi:10.1021/acs.chemrev.6b00193. hdl:1807/78811. PMID 27434758.
