 | |
| Names | |
|---|---|
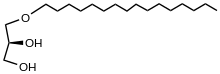
| IUPAC name
3-hexadecoxypropane-1,2-diol | |
| Other names
chimylic alcohol, 1-O-hexadecyl glycerol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C19H40O3 | |
| Molar mass | 316.526 g·mol−1 |
| Appearance | colorless solid |
| Melting point | 62.5–63.5 °C (144.5–146.3 °F; 335.6–336.6 K) |
| Boiling point | 445 °C (833 °F; 718 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Chimyl alcohol is an organic compound with the formula HOCH2CH(OH)CH2OC16H33. It is a colorless solid. Chimyl alcohol is a monoether formed by condensation of cetyl alcohol with one of the two primary alcohol sites of glycerol. Together with S-selachyl alcohol and S-batyl alcohol, S-chimyl alcohol is a component of some lipid membranes.[1] It is found in the liver of the shark Centrophorus squamosus.[2] The name chimyl is derived from a classification of ratfish, order Chimaeriformes. Like other glyceryl ethers, those derived from chimyl alcohol are not saponifiable.[3]
References
- ↑ Sutter, Marc; Silva, Eric Da; Duguet, Nicolas; Raoul, Yann; Métay, Estelle; Lemaire, Marc (2015). "Glycerol Ether Synthesis: A Bench Test for Green Chemistry Concepts and Technologies" (PDF). Chemical Reviews. 115 (16): 8609–8651. doi:10.1021/cr5004002. PMID 26196761.
- ↑ Bordier, Catherine G.; Sellier, Nicole; Foucault, Alain P.; Le Goffic, François (1996). "Purification and characterization of deep sea shark Centrophorus squamosus liver oil 1- O -aklylglycerol ether lipids". Lipids. 31 (5): 521–528. doi:10.1007/bf02522646. PMID 8727645. S2CID 39937991.
- ↑ Taguchi, Hiroyasu; Armarego, Wilfred L. F. (1998). "Glyceryl-Ether Monooxygenase [EC 1.14.16.5]. A Microsomal Enzyme of Ether Lipid Metabolism". Medicinal Research Reviews. 18 (1): 43–89. doi:10.1002/(SICI)1098-1128(199801)18:1<43::AID-MED3>3.0.CO;2-S. PMID 9436181. S2CID 432376.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.