
Compounds of lead exist with lead in two main oxidation states: +2 and +4. The former is more common. Inorganic lead(IV) compounds are typically strong oxidants or exist only in highly acidic solutions.[1]


_oxide.JPG.webp)
Chemistry
Various oxidized forms of lead are easily reduced to the metal. An example is heating PbO with mild organic reducing agents such as glucose. The mixture of the oxide and the sulfide heated together will also form the metal.[2]
- 2 PbO + PbS → 3 Pb + SO2
Metallic lead is attacked (oxidized) only superficially by air, forming a thin layer of lead oxide that protects it from further oxidation. The metal is not attacked by sulfuric or hydrochloric acids. It dissolves in nitric acid with the evolution of nitric oxide gas to form dissolved Pb(NO3)2.
- 3 Pb + 8 H+ + 8 NO−
3 → 3 Pb2+ + 6 NO−
3 + 2 NO + 4 H2O
When heated with nitrates of alkali metals, metallic lead oxidizes to form PbO (also known as litharge), leaving the corresponding alkali nitrite. PbO is representative of lead's +2 oxidation state. It is soluble in nitric and acetic acids, from which solutions it is possible to precipitate halide, sulfate, chromate, carbonate (PbCO3), and basic carbonate (Pb
3(OH)
2(CO
3)
2) salts of lead. The sulfide can also be precipitated from acetate solutions. These salts are all poorly soluble in water. Among the halides, the iodide is less soluble than the bromide, which, in turn, is less soluble than the chloride.[3]
Lead(II) oxide is also soluble in alkali hydroxide solutions to form the corresponding plumbite salt.[2]
- PbO + 2 OH− + H2O → Pb(OH)2−
4
Chlorination of plumbite solutions causes the formation of lead's +4 oxidation state.
- Pb(OH)2−
4 + Cl2 → PbO2 + 2 Cl− + 2 H2O
Lead dioxide is representative of the +4 oxidation state, and is a powerful oxidizing agent. The chloride of this oxidation state is formed only with difficulty and decomposes readily into lead(II) chloride and chlorine gas. The bromide and iodide of lead(IV) are not known to exist.[3] Lead dioxide dissolves in alkali hydroxide solutions to form the corresponding plumbates.[2]
- PbO2 + 2 OH− + 2 H2O → Pb(OH)2−
6
Lead also has an oxide with mixed +2 and +4 oxidation states, red lead (Pb
3O
4), also known as minium.
Lead readily forms an equimolar alloy with sodium metal that reacts with alkyl halides to form organometallic compounds of lead such as tetraethyllead.[4]
Oxides and sulfide
There are three oxides known: PbO, Pb3O4 (sometimes called "minium"), and PbO2. The former has two allotropes: α-PbO and β-PbO, both with layer structure and tetracoordinated lead. The alpha allotrope is red-colored and has the Pb–O distance of 230 pm; the beta allotrope is yellow-colored and has the Pb–O distance of 221 and 249 pm (due to asymmetry).[5] Thanks to the similarity, both allotropes can exist under standard conditions (beta with small (10−5 relative) impurities, such as Si, Ge, Mo, etc.). PbO reacts with acids to form salts, and with alkalies to give plumbites, [Pb(OH)3]− or [Pb(OH)4]2−.[6]
The dioxide may be prepared by, for example, halogenization of lead(II) salts. The alpha allotrope is rhombohedral, and the beta allotrope is tetragonal.[6] Both allotropes are black-brown in color and always contain some water, which cannot be removed, as heating also causes decomposition (to PbO and Pb3O4). The dioxide is a powerful oxidizer: it can oxidize hydrochloric and sulfuric acids. It does not reacts with alkaline solution, but reacts with solid alkalis to give hydroxyplumbates, or with basic oxides to give plumbates.[6]
Reaction of lead with sulfur or hydrogen sulfide yields lead sulfide. The solid has the NaCl-like structure (simple cubic), which it keeps up to the melting point, 1114 °C (2037 °F). If the heating occurs in presence of air, the compounds decomposes to give the monoxide and the sulfate.[7] The compounds are almost insoluble in water, weak acids, and (NH4)2S/(NH4)2S2 solution is the key for separation of lead from analytical groups I to III elements, tin, arsenic, and antimony. The compounds dissolve in nitric and hydrochloric acids, to give elemental sulfur and hydrogen sulfide, respectively.[7] Heating mixtures of the monoxide and the sulfide forms the metal.[2]
- 2 PbO + PbS → 3 Pb + SO2↑
Halides and other salts
Heating lead carbonate with hydrogen fluoride yields the hydrofluoride, which decomposes to the difluoride when it melts. This white crystalline powder is more soluble than the diiodide, but less than the dibromide and the dichloride. No coordinated lead fluorides exist (except the unstable PbF+ cation).[8] The tetrafluoride, a yellow crystalline powder, is unstable.
Other dihalides are received upon heating lead(II) salts with the halides of other metals; lead dihalides precipitate to give white orthorhombic crystals (diiodide form yellow hexagonal crystals). They can also be obtained by direct elements reaction at temperature exceeding melting points of dihalides. Their solubility increases with temperature; adding more halides first decreases the solubility, but then increases due to complexation, with the maximum coordination number being 6. The complexation depends on halide ion numbers, atomic number of the alkali metal, the halide of which is added, temperature and solution ionic strength.[9] The tetrachloride is obtained upon dissolving the dioxide in hydrochloric acid; to prevent the exothermic decomposition, it is kept under concentrated sulfuric acid. The tetrabromide may not, and the tetraiodide definitely does not exist.[10] The diastatide has also been prepared.[11]
The metal is not attacked by sulfuric or hydrochloric acids. It dissolves in nitric acid with the evolution of nitric oxide gas to form dissolved Pb(NO3)2.[8] It is a well-soluble solid in water; it is thus a key to receive the precipitates of halide, sulfate, chromate, carbonate, and basic carbonate Pb3(OH)2(CO3)2 salts of lead.[3]
Chloride complexes
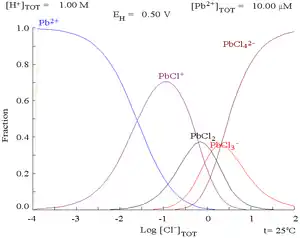
Lead(II) forms a series of complexes with chloride, the formation of which alters the corrosion chemistry of the lead. This will tend to limit the solubility of lead in saline media.
| Pb2+ + Cl− → PbCl+ | K1 = 12.59 |
| PbCl+ + Cl− → PbCl2 | K2 = 14.45 |
| PbCl2 + Cl− → PbCl− 3 |
K3 = 0.398 |
| PbCl− 3 + Cl− → PbCl2− 4 |
K4 = 0.0892 |
Organolead
The best-known compounds are the two simplest plumbane derivatives: tetramethyllead (TML) and tetraethyllead (TEL); however, the homologs of these, as well as hexaethyldilead (HEDL), are of lesser stability. The tetralkyl deratives contain lead(IV); the Pb–C bonds are covalent. They thus resemble typical organic compounds.[14]
Lead readily forms an equimolar alloy with sodium metal that reacts with alkyl halides to form organometallic compounds of lead such as tetraethyllead.[15] The Pb–C bond energies in TML and TEL are only 167 and 145 kJ/mol; the compounds thus decompose upon heating, with first signs of TEL composition seen at 100 °C (210 °F). Pyrolysis yields elemental lead and alkyl radicals; their interreaction causes the synthesis of HEDL.[14] They also decompose upon sunlight or UV-light.[16] In presence of chlorine, the alkyls begin to be replaced with chlorides; the R2PbCl2 in the presence of HCl (a by-product of the previous reaction) leads to the complete mineralization to give PbCl2. Reaction with bromine follows the same principle.[16]
Phase diagrams of solubilities
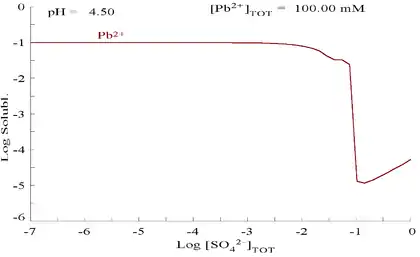
Lead(II) sulfate is poorly soluble, as can be seen in the following diagram showing addition of SO2−
4 to a solution containing 0.1 M of Pb2+. The pH of the solution is 4.5, as above that, Pb2+ concentration can never reach 0.1 M due to the formation of Pb(OH)2. Observe that Pb2+ solubility drops 10,000 fold as SO2−
4 reaches 0.1 M.
 |
 |
| Plot showing aqueous concentration of dissolved Pb2+ as a function of SO2− 4[12] |
Diagram for lead in sulfate media[12] |
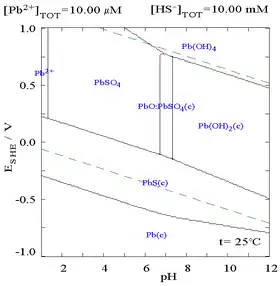
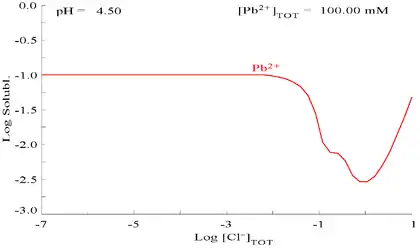
The addition of chloride can lower the solubility of lead, though in chloride-rich media (such as aqua regia) the lead can become soluble again as anionic chloro complexes.
 |
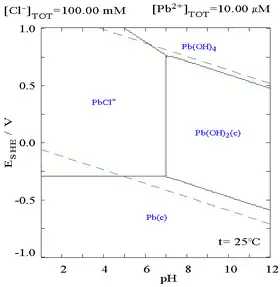 |
| Diagram showing the solubility of lead in chloride media. The lead concentrations are plotted as a function of the total chloride present.[12] | Pourbaix diagram for lead in chloride (0.1 M) media[12] |
References
- ↑ Polyanskiy 1986, pp. 14–15.
- 1 2 3 4 Pauling, Linus (1947). General Chemistry. W.H. Freeman. ISBN 978-0-486-65622-9.
- 1 2 3 Brady, James E.; Holum, John R. (1996). Descriptive Chemistry of the Elements. John Wiley and Sons. ISBN 978-0-471-13557-9.
- ↑ Windholz, Martha (1976). Merck Index of Chemicals and Drugs, 9th ed., monograph 8393. Merck. ISBN 978-0-911910-26-1.
- ↑ Polyanskiy 1986, p. 21.
- 1 2 3 Polyanskiy 1986, p. 22.
- 1 2 Polyanskiy 1986, p. 28.
- 1 2 Polyanskiy 1986, p. 32.
- ↑ Polyanskiy 1986, p. 33.
- ↑ Polyanskiy 1986, p. 34.
- ↑ Zuckerman, J. J.; Hagen, A. P. (1989). Inorganic Reactions and Methods, the Formation of Bonds to Halogens. John Wiley & Sons. p. 426. ISBN 978-0-471-18656-4.
- 1 2 3 4 5 Puigdomenech, Ignasi (2004). Hydra/Medusa Chemical Equilibrium Database and Plotting Software. KTH Royal Institute of Technology. Archived from the original on 2007-09-29.
- ↑ Ward, C. H.; Hlousek, Douglas A.; Phillips, Thomas A.; Lowe, Donald F. (2000). Remediation of Firing Range Impact Berms. CRC Press. ISBN 1566704626.
- 1 2 Polyanskiy 1986, p. 43.
- ↑ Windholz, Martha (1976). Merck Index of Chemicals and Drugs, 9th ed., monograph 8393. Merck. ISBN 0-911910-26-3.
- 1 2 Polyanskiy 1986, p. 44.
Bibliography
- Polyanskiy, N. G. (1986). Fillipova, N. A (ed.). Аналитическая химия элементов: Свинец [Analytical Chemistry of the Elements: Lead] (in Russian). Nauka.