 | |
| Names | |
|---|---|
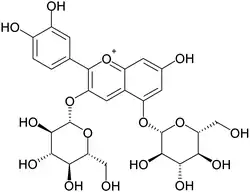
| IUPAC name
3,5-Bis(β-D-glucopyranosyloxy)-3′,4′,7-trihydroxyflavylium | |
| Systematic IUPAC name
2-(3,4-Dihydroxyphenyl)-7-hydroxy-3,5-bis{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-1λ4-benzopyran-1-ylium | |
| Other names
Cyanidin 3,5-O-diglucoside | |
| Identifiers | |
3D model (JSmol) |
|
| 1417221 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.018.214 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C27H31O16 | |
| Molar mass | 611.52 g/mol (chloride 647 g/mol) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Cyanidin-3,5-O-diglucoside, also known as cyanin, is an anthocyanin. It is the 3,5-O-diglucoside of cyanidin.
Natural occurrences
Cyanin can be found in species of the genus Rhaponticum (Asteraceae).[1]
In food
Cyanin can be found in red wine as well as pomegranate juice according to a study done by Graça Miguel, Susana Dandlen, Dulce Antunes, Alcinda Neves, and Denise Martins in the winter of 2004. Pomegranate juice extracted through centrifugal seed separation has higher amounts of cyanidin-3,5-O-diglucoside than juice extracted by squeezing fruit halves with an electric lemon squeezer.[2]
See also
References
- ↑ Vereskovskii, V. V.; Chekalinskaya, I. I. (July 1978). "Chrysanthemin and cyanin in species of the genus Rhaponticum". Chemistry of Natural Compounds. 14 (4): 450–451. doi:10.1007/bf00565267. S2CID 4817423.
- ↑ He, Fei; Liang, Na-Na; Mu, Lin; Pan, Qiu-Hong; Wang, Jun; Reeves, Michael J.; Duan, Chang-Qing (February 2012). "Anthocyanins and Their Variation in Red Wines I. Monomeric Anthocyanins and Their Color Expression". Molecules. 17 (2): 1571–1601. doi:10.3390/molecules17021571. PMC 6268338. PMID 22314380.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.