 | |
| Names | |
|---|---|
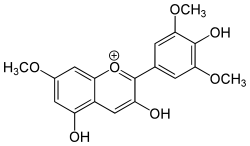
| IUPAC name
3,4′,5-Trihydroxy-3′,5′,7-trimethoxyflavylium | |
| Systematic IUPAC name
3,5-Dihydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)-7-methoxy-1λ4-benzopyran-1-ylium | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H17O7+ | |
| Molar mass | 345.32 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Hirsutidin is an O-methylated anthocyanidin, a chemical compound belonging to the anthocyanins. It can be found in Catharanthus roseus[1] (Madagascar periwinkle) where it is the prominent compound in petals and can also be found in callus cultures.[2]
Glycosides
3-O-(6-O-p-coumaroyl) glucoside of hirsutidin can also be found in Catharanthus roseus.[3]
References
- ↑ Characterization of the anthocyanins of Catharanthus roseus (L.) G. Don in vivo and in vitro by electrospray ionization ion trap mass spectrometry, Anna Piovan, Raffaella Filippini, Donata Favretto, 1998
- ↑ Catharanthus flavonoids on Schroeder page, uni. Freiburg, Germany
- ↑ Piovan, Anna; Filippini, Raffaella (2007). "Anthocyanins in Catharanthus roseus in vivo and in vitro: A review". Phytochemistry Reviews. 6 (2–3): 235–242. Bibcode:2007PChRv...6..235P. doi:10.1007/s11101-006-9052-y. S2CID 676724.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.