 | |
| Names | |
|---|---|
| Preferred IUPAC name
Cyclohexylbenzene | |
| Other names
Phenylcyclohexane | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.011.429 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H16 | |
| Molar mass | 160.260 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.982 g/cm3 |
| Melting point | 7.3 °C (45.1 °F; 280.4 K) |
| Boiling point | 240.1 °C (464.2 °F; 513.2 K) |
| Hazards | |
| GHS labelling: | |
  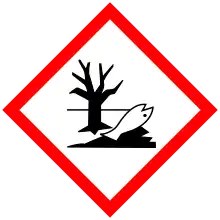 | |
| Danger | |
| H302, H304, H315, H319, H410 | |
| P264, P270, P273, P280, P301+P310, P301+P312, P302+P352, P305+P351+P338, P321, P330, P331, P332+P313, P337+P313, P362, P391, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
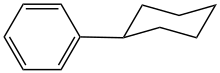
Cyclohexylbenzene is the organic compound with the structural formula C6H5−C6H11. It is a derivative of benzene with a cyclohexyl substituent (C6H11). It is a colorless liquid.
Formation
Cyclohexylbenzene is produced by the acid-catalyzed alkylation of benzene with cyclohexene.[1][2] The process can proceed using benzene as the exclusive organic precursor. Its partial hydrogenation gives cyclohexene, which alkylates the unhydrogenated benzene.[3]
It is also generated by the hydrodesulfurization of dibenzothiophene.[4]
Applications
A route to phenol analogous to the cumene process begins with cyclohexylbenzene, which is oxidized to a hydroperoxide, akin to the production of cumene hydroperoxide. Via the Hock rearrangement, cyclohexylbenzene hydroperoxide cleaves to give phenol and cyclohexanone:
- C6H5−C6H10OOH → C6H5OH + OC6H10
Cyclohexanone is an important precursor to some nylons.[5][3]
References
- ↑ Qiao, Kun; Yokoyama, Chiaki (2004). "Novel Acidic Ionic Liquids Catalytic Systems for Friedel–Crafts Alkylation of Aromatic Compounds with Alkenes". Chemistry Letters. 33 (4): 472–473. doi:10.1246/cl.2004.472.
- ↑ B. B. Corson, V. N. Ipatieff (1939). "Cyclohexylbenzene". Organic Syntheses. 19: 36. doi:10.15227/orgsyn.019.0036.
- 1 2 Weber, Manfred; Weber, Markus; Kleine-Boymann, Michael (2004). "Phenol". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_299.pub3. ISBN 978-3527306732.
- ↑ Bai, Jin; Li, Xiang; Wang, Anjie; Prins, Roel; Wang, Yao (2012). "Hydrodesulfurization of Dibenzothiophene and its Hydrogenated Intermediates over Bulk MoP". Journal of Catalysis. 287: 161–169. doi:10.1016/j.jcat.2011.12.018.
- ↑ Plotkin, Jeffrey S. (2016-03-21). "What's New in Phenol Production?". American Chemical Society. Archived from the original on 2019-10-27. Retrieved 2018-01-02.