 | |
| Names | |
|---|---|
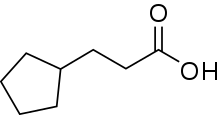
| Preferred IUPAC name
3-Cyclopentylpropanoic acid | |
| Other names
3-Cyclopentylpropionic acid; 3-Cyclopentanepropionic acid; Cypionate; Cipionate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.004.940 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H14O2 | |
| Molar mass | 142.198 g·mol−1 |
| Density | 0.996 g/mL[1] |
| Melting point | 130 to 132 °C (266 to 270 °F; 403 to 405 K) (12 mmHg) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Cypionic acid, also known as cyclopentylpropionic acid, is an aliphatic carboxylic acid with the molecular formula C8H14O2. Its salts and esters are known as cypionates or cipionates.
The primary use of cypionic acid is in pharmaceutical formulations. Cypionic acid is used to prepare ester prodrugs which have increased half-lives relative to the parent compound. The lipophilicity of the cypionate group allows the prodrug to be sequestered in fat depots after intramuscular injection.[2] The ester group is slowly hydrolyzed by metabolic enzymes, releasing steady doses of the active ingredient. Examples include testosterone cypionate, estradiol cypionate, hydrocortisone cypionate, oxabolone cipionate, and mesterolone cypionate.
References
- ↑ 3-Cyclopentylpropionic acid at Sigma-Aldrich
- ↑ VJ. Stella, W.N A. Charman and V.H. Naringrekar (1985). "Prodrugs: Do They Have Advantages in Clinical Practice?". Drugs. 29 (5): 455–473. doi:10.2165/00003495-198529050-00002. PMID 3891303.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.