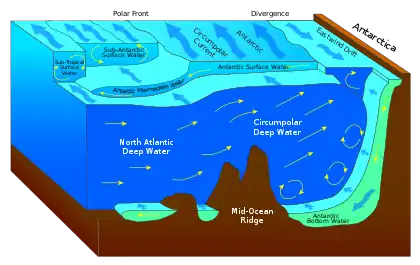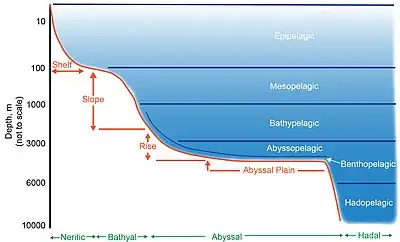
The deep sea is broadly defined as the ocean depth where light begins to fade, at an approximate depth of 200 m (660 ft) or the point of transition from continental shelves to continental slopes.[1][2] Conditions within the deep sea are a combination of low temperatures, darkness, and high pressure.[3] The deep sea is considered the least explored Earth biome as the extreme conditions make the environment difficult to access and explore.[4]
Organisms living within the deep sea have a variety of adaptations to survive in these conditions.[5] Organisms can survive in the deep sea through a number of feeding methods including scavenging, predation and filtration, with a number of organisms surviving by feeding on marine snow.[6] Marine snow is organic material that has fallen from upper waters into the deep sea.[7]
In 1960, the bathyscaphe Trieste descended to the bottom of the Mariana Trench near Guam, at 10,911 m (35,797 ft; 6.780 mi), the deepest known spot in any ocean. If Mount Everest (8,848 m or 29,029 ft or 5.498 mi) were submerged there, its peak would be more than 2 km (1.2 mi) beneath the surface. After the Trieste was retired, the Japanese remote-operated vehicle (ROV) Kaikō was the only vessel capable of reaching this depth until it was lost at sea in 2003.[8] In May and June 2009, the hybrid-ROV Nereus returned to the Challenger Deep for a series of three dives to depths exceeding 10,900 m (35,800 ft; 6.8 mi).
Environmental characteristics
Light
Natural light does not penetrate the deep ocean, with the exception of the upper parts of the mesopelagic. Since photosynthesis is not possible, plants and phytoplankton cannot live in this zone, and as these are the primary producers of almost all of earth's ecosystems, life in this area of the ocean must depend on energy sources from elsewhere. Except for the areas close to the hydrothermal vents, this energy comes from organic material drifting down from the photic zone. The sinking organic material is composed of algal particulates, detritus, and other forms of biological waste, which is collectively referred to as marine snow.
Pressure
Because pressure in the ocean increases by about 1 atmosphere for every 10 meters of depth, the amount of pressure experienced by many marine organisms is extreme. Until recent years, the scientific community lacked detailed information about the effects of pressure on most deep sea organisms because the specimens encountered arrived at the surface dead or dying and weren't observable at the pressures at which they lived. With the advent of traps that incorporate a special pressure-maintaining chamber, undamaged larger metazoan animals have been retrieved from the deep sea in good condition.
Salinity
Salinity is remarkably constant throughout the deep sea, at about 35 parts per thousand.[9] There are some minor differences in salinity, but none that are ecologically significant, except in largely landlocked seas like the Mediterranean and Red Seas.
Temperature
The two areas of greatest temperature gradient in the oceans are the transition zone between the surface waters and the deep waters, the thermocline, and the transition between the deep-sea floor and the hot water flows at the hydrothermal vents. Thermoclines vary in thickness from a few hundred meters to nearly a thousand meters. Below the thermocline, the water mass of the deep ocean is cold and far more homogeneous. Thermoclines are strongest in the tropics, where the temperature of the epipelagic zone is usually above 20 °C. From the base of the epipelagic, the temperature drops over several hundred meters to 5 or 6 °C at 1,000 meters. It continues to decrease to the bottom, but the rate is much slower. The cold water stems from sinking heavy surface water in the polar regions.[9]
At any given depth, the temperature is practically unvarying over long periods of time, without seasonal changes and with very little interannual variability. No other habitat on earth has such a constant temperature.[10]
In hydrothermal vents the temperature of the water as it emerges from the "black smoker" chimneys may be as high as 400 °C (it is kept from boiling by the high hydrostatic pressure) while within a few meters it may be back down to 2 to 4 °C.[11]
Biology
Regions below the epipelagic are divided into further zones, beginning with the bathyal zone (also considered the continental slope) which spans from 200 to 3000 meters[12] below sea level and is essentially transitional, containing elements from both the shelf above and the abyss below.[13] Below this zone, the deep sea consists of the abyssal zone which occurs between the ocean depths of 3000 and 6000 meters[14] and the hadal zone (6000 – 11,000 meters).[15][16] Food consists of falling organic matter known as 'marine snow' and carcasses derived from the productive zone above, and is scarce both in terms of spatial and temporal distribution.[17]
Instead of relying on gas for their buoyancy, many deep-sea species have jelly-like flesh consisting mostly of glycosaminoglycans, which provides them with very low density. It is also common among deep water squid to combine the gelatinous tissue with a flotation chamber filled with a coelomic fluid made up of the metabolic waste product ammonium chloride, which is lighter than the surrounding water.
The midwater fish have special adaptations to cope with these conditions—they are small, usually being under 25 centimetres (10 in); they have slow metabolisms and unspecialized diets, preferring to sit and wait for food rather than waste energy searching for it. They have elongated bodies with weak, watery muscles and skeletal structures. They often have extendable, hinged jaws with recurved teeth. Because of the sparse distribution and lack of light, finding a partner with which to breed is difficult, and many organisms are hermaphroditic.
Because light is so scarce, fish often have larger than normal, tubular eyes with only rod cells.[18][19] Their upward field of vision allows them to seek out the silhouette of possible prey.[20] Prey fish however also have adaptations to cope with predation. These adaptations are mainly concerned with reduction of silhouettes, a form of camouflage. The two main methods by which this is achieved are reduction in the area of their shadow by lateral compression of the body,[21] and counter illumination via bioluminescence.[22][19] This is achieved by production of light from ventral photophores, which tend to produce such light intensity to render the underside of the fish of similar appearance to the background light. For more sensitive vision in low light, some fish have a retroreflector behind the retina.[23] Flashlight fish have this plus photophores, which combination they use to detect eyeshine in other fish (see tapetum lucidum).[24][25]
Organisms in the deep sea are almost entirely reliant upon sinking living and dead organic matter which falls at approximately 100 meters per day.[26] In addition, only about 1 to 3% of the production from the surface reaches the sea bed mostly in the form of marine snow. Larger food falls, such as whale carcasses, also occur and studies have shown that these may happen more often than currently believed. There are many scavengers that feed primarily or entirely upon large food falls and the distance between whale carcasses is estimated to only be 8 kilometers.[27] In addition, there are a number of filter feeders that feed upon organic particles using tentacles, such as Freyella elegans.[28]
Marine bacteriophages play an important role in cycling nutrients in deep sea sediments. They are extremely abundant (between 5×1012 and 1×1013 phages per square meter) in sediments around the world.[29]
Despite being so isolated deep sea organisms have still been harmed by human interaction with the oceans. The London Convention[30] aims to protect the marine environment from dumping of wastes such as sewage sludge[31] and radioactive waste. A study found that at one region there had been a decrease in deep sea coral from 2007 to 2011, with the decrease being attributed to global warming and ocean acidification, and biodiversity estimated as being at the lowest levels in 58 years.[32] Ocean acidification is particularly harmful to deep sea corals because they are made of aragonite, an easily soluble carbonate, and because they are particularly slow growing and will take years to recover.[33] Deep sea trawling is also harming the biodiversity by destroying deep sea habitats which can take years to form.[34] Another human activity that has altered deep sea biology is mining. One study found that at one mining site fish populations had decreased at six months and at three years, and that after twenty six years populations had returned to the same levels as prior to the disturbance.[35]
Chemosynthesis
There are a number of species that do not primarily rely upon dissolved organic matter for their food. These species and communities are found at hydrothermal vents at sea-floor spreading zones.[36][37] One example is the symbiotic relationship between the tube worm Riftia and chemosynthetic bacteria.[38] It is this chemosynthesis that supports the complex communities that can be found around hydrothermal vents. These complex communities are one of the few ecosystems on the planet that do not rely upon sunlight for their supply of energy.[39]
Adaptation to hydrostatic pressure
Deep sea fish have different adaptations in their proteins, anatomical structures, and metabolic systems to survive in the Deep sea, where the inhabitants have to withstand great amount of hydrostatic pressure. While other factors like food availability and predator avoidance are important, the deep-sea organisms must have the ability to maintain well-regulated metabolic system in the face of high pressures.[40] In order to adjust for the extreme environment, these organisms have developed unique characteristics.
Proteins are affected greatly by the elevated hydrostatic pressure, as they undergo changes in water organization during hydration and dehydration reactions of the binding events. This is due to the fact that most enzyme-ligand interactions form through charged or polar non-charge interactions. Because hydrostatic pressure affects both protein folding and assembly and enzymatic activity, the deep sea species must undergo physiological and structural adaptations to preserve protein functionality against pressure.[40][41]
Actin is a protein that is essential for different cellular functions. The α-actin serves as a main component for muscle fiber, and it is highly conserved across numerous different species. Some Deep-sea fish developed pressure tolerance through the change in mechanism of their α-actin. In some species that live in depths greater than 5000m, C.armatus and C.yaquinae have specific substitutions on the active sites of α-Actin, which serves as the main component of muscle fiber.[42] These specific substitutions, Q137K and V54A from C.armatus or I67P from C.yaquinae are predicted to have importance in pressure tolerance.[42] Substitution in the active sites of actin result in significant changes in the salt bridge patterns of the protein, which allows for better stabilization in ATP binding and sub unit arrangement, confirmed by the free energy analysis and molecular dynamics simulation.[43] It was found that deep sea fish have more salt bridges in their actins compared to fish inhabiting the upper zones of the sea.[42]
In relations to protein substitution, specific osmolytes were found to be abundant in deep sea fish under high hydrostatic pressure. For certain chondrichtyans, it was found that Trimethylamine N-oxide (TMAO) increased with depth, replacing other osmolytes and urea.[44] Due to the ability of TMAO being able to protect proteins from high hydrostatic pressure destabilizing proteins, the osmolyte adjustment serves are an important adaptation for deep sea fish to withstand high hydrostatic pressure.
Deep-sea organisms possess molecular adaptations to survive and thrive in the deep oceans. Mariana hadal snailfish developed modification in the Osteocalcin(burlap) gene, where premature termination of the gene was found.[41] Osteocalcin gene regulates bone development and tissue mineralization, and the frameshift mutation seems to have resulted in the open skull and cartilage-based bone formation.[41] Due to high hydrostatic pressure in the deep sea, closed skulls that organisms living on the surface develop cannot withstand the enforcing stress. Similarly, common bone developments seen in surface vertebrates cannot maintain their structural integrity under constant high pressure.[41]
Exploration
It has been suggested that more is known about the Moon than the deepest parts of the ocean.[45] This is a common misconception based on a 1953 statement by George E.R. Deacon published in the Journal of Navigation, and largely refers to the scarce amount of seafloor bathymetry available at the time.[46] The similar idea that more people have stood on the moon than have been to the deepest part of the ocean is likewise problematic and dangerous.[46]
Still the deep-sea remains one of the least explored regions on planet Earth.[47] Pressures even in the mesopelagic become too great for traditional exploration methods, demanding alternative approaches for deep-sea research. Baited camera stations, small manned submersibles, and ROVs (remotely operated vehicles) are three methods utilized to explore the ocean's depths. Because of the difficulty and cost of exploring this zone, current knowledge is limited. Pressure increases at approximately one atmosphere for every 10 meters meaning that some areas of the deep sea can reach pressures of above 1,000 atmospheres. This not only makes great depths very difficult to reach without mechanical aids, but also provides a significant difficulty when attempting to study any organisms that may live in these areas as their cell chemistry will be adapted to such vast pressures.
See also
- Deep ocean water – Cold, salty water deep below the surface of Earth's oceans
- Submarine landslide – Landslides that transport sediment across the continental shelf and into the deep ocean
- The Blue Planet – 2001 British nature documentary television series
- Blue Planet II – 2017 British nature documentary television series
- Nepheloid layer – layer of water in the deep ocean basin, above the ocean floor, that contains significant amounts of suspended sediment
- Biogenous ooze
 Oceans portal
Oceans portal
References
- ↑ Tyler, P. A. (2003). In Ecosystems of the World 28, Ecosystems of the Deep Sea. Amsterdam: Elsevier. pp. 1–3.
- ↑ "What is the "deep" ocean? : Ocean Exploration Facts: NOAA Office of Ocean Exploration and Research". oceanexplorer.noaa.gov. Retrieved 2022-09-29.
- ↑ Paulus, Eva (2021). "Shedding Light on Deep-Sea Biodiversity—A Highly Vulnerable Habitat in the Face of Anthropogenic Change". Frontiers in Marine Science. 8. doi:10.3389/fmars.2021.667048. ISSN 2296-7745.
- ↑ Danovaro, Roberto; Corinaldesi, Cinzia; Dell’Anno, Antonio; Snelgrove, Paul V. R. (2017-06-05). "The deep-sea under global change". Current Biology. 27 (11): R461–R465. doi:10.1016/j.cub.2017.02.046. ISSN 0960-9822. PMID 28586679. S2CID 20785268.
- ↑ Brown, Alastair; Thatje, Sven (2013). "Explaining bathymetric diversity patterns in marine benthic invertebrates and demersal fishes: physiological contributions to adaptation of life at depth". Biological Reviews. 89 (2): 406–426. doi:10.1111/brv.12061. ISSN 1464-7931. PMC 4158864. PMID 24118851.
- ↑ Higgs, Nicholas D.; Gates, Andrew R.; Jones, Daniel O. B. (2014-05-07). "Fish Food in the Deep Sea: Revisiting the Role of Large Food-Falls". PLOS ONE. 9 (5): e96016. Bibcode:2014PLoSO...996016H. doi:10.1371/journal.pone.0096016. ISSN 1932-6203. PMC 4013046. PMID 24804731.
- ↑ US Department of Commerce, National Oceanic and Atmospheric Administration. "What is marine snow?". oceanservice.noaa.gov. Retrieved 2022-09-29.
- ↑ Horstman, Mark (2003-07-09). "Hope floats for lost deep-sea explorer". www.abc.net.au. Archived from the original on 2010-09-27. Retrieved 2021-05-07.
- 1 2 Claus Detlefsen. "About the Marianas" (in Danish) Ingeniøren / Geological Survey of Denmark and Greenland, 2 November 2013. Accessed: 2 November 2013.
- ↑ MarineBio (2018-06-17). "The Deep Sea". MarineBio Conservation Society. Retrieved 2020-08-07.
- ↑ Nybakken, James W. Marine Biology: An Ecological Approach. Fifth Edition. Benjamin Cummings, 2001. p. 136 - 141.
- ↑ Levin, Lisa A.; Dayton, Paul K. (1 November 2009). "Ecological theory and continental margins: where shallow meets deep". Trends in Ecology & Evolution. 24 (11): 606–617. doi:10.1016/j.tree.2009.04.012. ISSN 0169-5347. PMID 19692143. Retrieved 29 September 2022.
- ↑ Gage, John D.; Tyler, Paul A. (18 April 1991). Deep-Sea Biology: A Natural History of Organisms at the Deep-Sea Floor. Cambridge University Press. ISBN 978-0-521-33431-0. Retrieved 29 September 2022.
- ↑ Smith, Craig R.; De Leo, Fabio C.; Bernardino, Angelo F.; Sweetman, Andrew K.; Arbizu, Pedro Martinez (1 September 2008). "Abyssal food limitation, ecosystem structure and climate change". Trends in Ecology & Evolution. 23 (9): 518–528. doi:10.1016/j.tree.2008.05.002. ISSN 0169-5347. PMID 18584909. Retrieved 29 September 2022.
- ↑ Jamieson, Alan J.; Fujii, Toyonobu; Mayor, Daniel J.; Solan, Martin; Priede, Imants G. (1 March 2010). "Hadal trenches: the ecology of the deepest places on Earth". Trends in Ecology & Evolution. 25 (3): 190–197. doi:10.1016/j.tree.2009.09.009. ISSN 0169-5347. PMID 19846236. Retrieved 29 September 2022.
- ↑ Jamieson, Alan J.; Stewart, Heather A.; Weston, Johanna N. J.; Lahey, Patrick; Vescovo, Victor L. (2022). "Hadal Biodiversity, Habitats and Potential Chemosynthesis in the Java Trench, Eastern Indian Ocean". Frontiers in Marine Science. 9. doi:10.3389/fmars.2022.856992.
- ↑ Robison, Bruce H.; Reisenbichler, Kim R.; Sherlock, Rob E. (2005-06-10). "Giant Larvacean Houses: Rapid Carbon Transport to the Deep Sea Floor". Science. 308 (5728): 1609–1611. Bibcode:2005Sci...308.1609R. doi:10.1126/science.1109104. ISSN 0036-8075. PMID 15947183. S2CID 730130.
- ↑ Lupše, Nik; Cortesi, Fabio; Freese, Marko; Marohn, Lasse; Pohlmann, Jan-Dag; Wysujack, Klaus; Hanel, Reinhold; Musilova, Zuzana (9 December 2021). "Visual Gene Expression Reveals a cone-to-rod Developmental Progression in Deep-Sea Fishes". Molecular Biology and Evolution. 38 (12): 5664–5677. doi:10.1093/molbev/msab281. PMC 8662630. PMID 34562090. Retrieved 29 September 2022.
- 1 2 Warrant, Eric J.; Locket, N. Adam (August 2004). "Vision in the deep sea". Biological Reviews. 79 (3): 671–712. doi:10.1017/S1464793103006420. ISSN 1469-185X. PMID 15366767. S2CID 34907805.
- ↑ Collin, Shaun P.; Chapuis, Lucille; Michiels, Nico K. (2019). "Impacts of (extreme) depth on life in the deep-sea". Marine Extremes. Routledge. pp. 197–216. doi:10.4324/9780429491023-12. ISBN 9780429491023. S2CID 133939260. Retrieved 29 September 2022.
- ↑ Hoving, Henk‐Jan T.; Freitas, Rui (February 2022). "Pelagic observations of the midwater scorpionfish Ectreposebastes imus (Setarchidae) suggests a role in trophic coupling between deep‐sea habitats". Journal of Fish Biology. 100 (2): 586–589. doi:10.1111/jfb.14944. PMID 34751439. S2CID 243863469. Retrieved 29 September 2022.
- ↑ Davis, Alexander L.; Sutton, Tracey T.; Kier, William M.; Johnsen, Sönke (10 June 2020). "Evidence that eye-facing photophores serve as a reference for counterillumination in an order of deep-sea fishes". Proceedings of the Royal Society B: Biological Sciences. 287 (1928): 20192918. doi:10.1098/rspb.2019.2918. PMC 7341941. PMID 32517614.
- ↑ Palmer, Benjamin A.; Gur, Dvir; Weiner, Steve; Addadi, Lia; Oron, Dan (October 2018). "The Organic Crystalline Materials of Vision: Structure-Function Considerations from the Nanometer to the Millimeter Scale". Advanced Materials. 30 (41): 1800006. doi:10.1002/adma.201800006. PMID 29888511. S2CID 47005095. Retrieved 29 September 2022.
- ↑ Hellinger, Jens; Jägers, Peter; Donner, Marcel; Sutt, Franziska; Mark, Melanie D.; Senen, Budiono; Tollrian, Ralph; Herlitze, Stefan (2017-02-08). De, Abhijit (ed.). "The Flashlight Fish Anomalops katoptron Uses Bioluminescent Light to Detect Prey in the Dark". PLOS ONE. 12 (2): e0170489. Bibcode:2017PLoSO..1270489H. doi:10.1371/journal.pone.0170489. ISSN 1932-6203. PMC 5298212. PMID 28178297.
- ↑ Cavallaro, Mauro; Guerrera, Maria Cristina; Abbate, Francesco; Levanti, Maria Beatrice; Laurà, Rosaria; Ammendolia, Giovanni; Malara, Danilo; Stipa, Maria Giulia; Battaglia, Pietro (October 2021). "Morphological, ultrastructural and immunohistochemical study on the skin ventral photophores of Diaphus holti Tåning, 1918 (Family: Myctophidae)". Acta Zoologica. 102 (4): 405–411. doi:10.1111/azo.12348. ISSN 0001-7272. S2CID 225368866.
- ↑ "Marine Snow and Fecal Pellets". Oceanus Magazine.
- ↑ R. N. Gibson, Harold (CON) Barnes, R. J. A. Atkinson, Oceanography and Marine Biology, An Annual Review. 2007. Volume 41. Published by CRC Press, 2004 ISBN 0-415-25463-9, ISBN 978-0-415-25463-2
- ↑ "Discover - Natural History Museum". www.nhm.ac.uk.
- ↑ Danovaro, Roberto; Antonio Dell'Anno; Cinzia Corinaldesi; Mirko Magagnini; Rachel Noble; Christian Tamburini; Markus Weinbauer (2008-08-28). "Major viral impact on the functioning of benthic deep-sea ecosystems". Nature. 454 (7208): 1084–1087. Bibcode:2008Natur.454.1084D. doi:10.1038/nature07268. PMID 18756250. S2CID 4331430.
- ↑ "London Convention". International Maritime Organization. Archived from the original on 3 March 2019. Retrieved 24 March 2020.
- ↑ Snelgrove, Paul; Grassle, Fred (1995-01-01). "What of the deep sea's future diversity?". Oceanus. 38 (2). Retrieved 24 March 2020.
- ↑ Zimmerman, Alexander N.; Johnson, Claudia C.; Bussberg, Nicholas W.; Dalkilic, Mehmet M. (April 2020). "Stability and decline in deep-sea coral biodiversity, Gulf of Mexico and US West Atlantic". Coral Reefs. 39 (2): 345–359. doi:10.1007/s00338-020-01896-9. S2CID 210975434.
- ↑ Ruttimann, Jacqueline (2006-08-31). "Oceanography: sick seas". Nature. 442 (7106): 978–80. Bibcode:2006Natur.442..978R. doi:10.1038/442978a. PMID 16943816. S2CID 4332965.
- ↑ Koslow, Tony (2011-11-20). "The silent deep: the discovery, ecology & conservation of the deep sea". Pacific Ecologist. 20.
- ↑ Drazen, Jeffery; Leitner, Astrid; Morningstar, Sage; Marcon, Yann; Greinert, Jens; Purser, Auntun (2019-01-01). "Observations of deep-sea fishes and mobile scavengers from the abyssal DISCOL experimental mining area". Biogeosciences. 16 (16): 3133–3146. Bibcode:2019BGeo...16.3133D. doi:10.5194/bg-16-3133-2019. ProQuest 2276806480.
- ↑ Dover, Cindy Van (2000-03-26). The Ecology of Deep-Sea Hydrothermal Vents. Princeton University Press. ISBN 978-0-691-04929-8.
- ↑ Martin, William; Baross, John; Kelley, Deborah; Russell, Michael J. (November 2008). "Hydrothermal vents and the origin of life". Nature Reviews Microbiology. 6 (11): 805–814. doi:10.1038/nrmicro1991. ISSN 1740-1534. PMID 18820700. S2CID 1709272.
- ↑ Cavanaugh, F. J. Stewart and C. M. (2006). "Symbiosis of Thioautotrophic Bacteria with Riftia pachyptila | EndNote Click". Progress in Molecular and Subcellular Biology. 41: 197–225. doi:10.1007/3-540-28221-1_10. PMID 16623395. Retrieved 2022-09-29.
- ↑ HW Jannasch. 1985. The Chemosynthetic Support of Life and the Microbial Diversity at Deep-Sea Hydrothermal Vents. Proceedings of the Royal Society of London. Series B, Biological Sciences, Vol. 225, No. 1240 (Sep. 23, 1985), pp. 277-297
- 1 2 "Chapter Twelve. Adaptations to the Deep Sea", Biochemical Adaptation, Princeton University Press, pp. 450–495, 1984-12-31, doi:10.1515/9781400855414.450, ISBN 978-1-4008-5541-4, retrieved 2020-11-02
- 1 2 3 4 Wang, Kun; Shen, Yanjun; Yang, Yongzhi; Gan, Xiaoni; Liu, Guichun; Hu, Kuang; Li, Yongxin; Gao, Zhaoming; Zhu, Li; Yan, Guoyong; He, Lisheng (May 2019). "Morphology and genome of a snailfish from the Mariana Trench provide insights into deep-sea adaptation". Nature Ecology & Evolution. 3 (5): 823–833. doi:10.1038/s41559-019-0864-8. ISSN 2397-334X. PMID 30988486.
- 1 2 3 Wakai, Nobuhiko; Takemura, Kazuhiro; Morita, Takami; Kitao, Akio (2014-01-20). "Mechanism of Deep-Sea Fish α-Actin Pressure Tolerance Investigated by Molecular Dynamics Simulations". PLOS ONE. 9 (1): e85852. Bibcode:2014PLoSO...985852W. doi:10.1371/journal.pone.0085852. ISSN 1932-6203. PMC 3896411. PMID 24465747.
- ↑ Hata, Hiroaki; Nishiyama, Masayoshi; Kitao, Akio (2020-02-01). "Molecular dynamics simulation of proteins under high pressure: Structure, function and thermodynamics". Biochimica et Biophysica Acta (BBA) - General Subjects. Novel measurement techniques for visualizing 'live' protein molecules. 1864 (2): 129395. doi:10.1016/j.bbagen.2019.07.004. ISSN 0304-4165. PMID 31302180. S2CID 196613044.
- ↑ Yancey, Paul H.; Speers-Roesch, Ben; Atchinson, Sheila; Reist, James D.; Majewski, Andrew R.; Treberg, Jason R. (2017-11-27). "Osmolyte Adjustments as a Pressure Adaptation in Deep-Sea Chondrichthyan Fishes: An Intraspecific Test in Arctic Skates (Amblyraja hyperborea) along a Depth Gradient". Physiological and Biochemical Zoology. 91 (2): 788–796. doi:10.1086/696157. ISSN 1522-2152. PMID 29315031. S2CID 26847773.
- ↑ Tim Flannery, Where Wonders Await Us. New York Review of Books, December 2007
- 1 2 Jamieson, Alan J; Singleman, Glenn; Linley, Thomas D; Casey, Susan (2020-12-21). "Fear and loathing of the deep ocean: why don't people care about the deep sea?". ICES Journal of Marine Science. 78 (3): 797–809. doi:10.1093/icesjms/fsaa234. ISSN 1054-3139.
- ↑ Briand, F.; Snelgrove, P. (2003). "Mare Incognitum? An overview". CIESM Workshop Monographs. 23: 5–27.
External links
- Deep Sea Foraminifera – Deep Sea Foraminifera from 4400 meters depth, Antarctica - an image gallery and description of hundreds of specimens
- Deep Ocean Exploration on the Smithsonian Ocean Portal
- Deep-Sea Creatures Facts and images from the deepest parts of the ocean
- How Deep Is The Ocean Archived 2016-06-15 at the Wayback Machine Facts and infographic on ocean depth