 | |
 | |
| Names | |
|---|---|
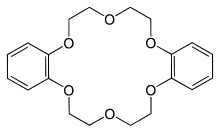
| Preferred IUPAC name
6,7,9,10,17,18,20,21-Octahydrodibenzo[b,k][1,4,7,10,13,16]hexaoxacyclooctadecine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.034.568 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H24O6 | |
| Molar mass | 360.406 g·mol−1 |
| Appearance | white solid |
| Melting point | 162.5–163.5 °C (324.5–326.3 °F; 435.6–436.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Dibenzo-18-crown-6 is the organic compound with the formula [OC6H4OCH2CH2OCH2CH2]2. It is a white solid that is soluble in organic solvents. As one of the most popular crown ethers, it facilitates the dissolution of many salts in organic solvents. It is related to the non-benzannulated 18-crown-6.
Dibenzo-18-crown-6 can be synthesized from catechol and bis(chloroethyl) ether.[1]
In contrast to those of 18-crown-6, complexes of the dibenzo crown are flattened, which often allows higher coordination numbers at the encapsulated metal cation.
FeCl4_(TITWIC).png.webp)
Structure of [Na(dibenzo-18-c-6)]+, as found in the FeCl4− salt.[2]
References
- ↑ Charles J. Pedersen (1972). "Macrocyclic Polyethers: Dibenzo-18-Crown-6 Polyether and Dicyclohexyl-18-Crown-6 Polyether". Organic Syntheses. 52: 66. doi:10.15227/orgsyn.052.0066.
- ↑ Rogers, Robin D.; Song, Ying (1995). "The crystal structure of a heterobimetallic crown ether complex: [Na(dibenzo-18-crown-6)][FeCl4]". Journal of Chemical Crystallography. 25 (9): 579–582. doi:10.1007/BF01667027. S2CID 93955378.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
