 | |
| Names | |
|---|---|
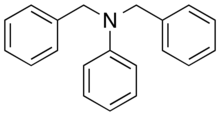
| Preferred IUPAC name
N,N-Dibenzylaniline | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H19N | |
| Molar mass | 273.379 g·mol−1 |
| Appearance | yellowish white crystals |
| Melting point | 69.0 °C (156.2 °F; 342.1 K) |
| Boiling point | 300 °C (572 °F; 573 K) above |
| insol | |
| Solubility | ether, ethanol |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H312, H315, H319 | |
| P264, P270, P280, P301+P312, P302+P352, P305+P351+P338, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Dibenzylaniline or N,N-Dibenzylaniline is a chemical compound consisting of aniline with two benzyl groups as substituents on the nitrogen.
The substance crystallizes in the monoclinic crystal system. The space group is P21/n. The unit cell dimensions are a=11.751 Å b=9.060 Å c=29.522 Å, and β=94.589°.[1] Each unit cell contains two molecules. In the solid van der Waals forces hold it together.[2] The substance can also crystallize in alternate monoclinic form.[3]
Production
One method to produce dibenzylaniline is using a mixture of dibutyl tin dichloride and dibutyl stannane with N-benzilideneaniline along with hexamethylphosphoric triamide dissolved in tetrahydrofuran which yields a tin amide compound. This then reacts with benzyl bromide to yield dibenzylaniline.[4]
Another method uses aniline and benzyl bromide.
Use
It used to make dyes.
A nitroso derivative (made using nitrite and hydroxylamine) can be used in a colourimetric test for palladium.[5]
References
- ↑ Bi, Quan-Xi; Tong, Hong-Bo; Zhou, Mei-Su (2007). "Crystal Structure". CCDC 642961: Experimental Crystal Structure Determination. Cambridge Crystallographic Data Centre. doi:10.5517/ccpl1pz.
- ↑ Bi, Quan-Xi; Tong, Hong-Bo; Zhou, Mei-Su (21 March 2007). "N,N-Dibenzylaniline". Acta Crystallographica Section E. 63 (4): o1809–o1810. Bibcode:2007AcCrE..63O1809B. doi:10.1107/S1600536807011476.
- ↑ Tong, Hong-Bo; Zhou, Mei-Su; Bi, Quan-Xi; Chao, Jian-Bin (7 November 2007). "A new polymorph of N,N-dibenzylaniline". Acta Crystallographica Section E. 63 (12): o4560. Bibcode:2007AcCrE..63O4560T. doi:10.1107/S1600536807054402.
- ↑ Fürstner, Alois (2014). Science of Synthesis Knowledge Updates 2013. Georg Thieme Verlag. pp. 72–73. ISBN 978-3-13-178881-8.
- ↑ Brooks, Robert R. (1992). Noble Metals and Biological Systems: Their Role in Medicine, Mineral Exploration, and the Environment. CRC Press. p. 142. ISBN 978-0-8493-6164-7.