 | |
| Names | |
|---|---|
| IUPAC name
2,2-dichloroethanal | |
| Other names
dichloroethanal | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ECHA InfoCard | 100.001.063 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
| UN number | 1993 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H2Cl2O | |
| Molar mass | 112.94 g·mol−1 |
| Density | 1.4 g/mL |
| Melting point | −50 °C (−58 °F; 223 K) |
| Boiling point | 88 °C (190 °F; 361 K) |
| forms hydrate | |
| Related compounds | |
Related compounds |
chloroacetaldehyde, trichloroacetaldehyde |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
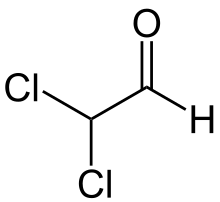
Dichloroacetaldehyde is a chlorinated aldehyde with the chemical formula HCCl2CHO. Along with monochloroacetaldehyde and trichloroacetaldehyde, it is one of the three possible chlorinated acetaldehydes.
Properties and reactions
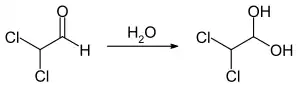
Dichloroacetaldehyde is a highly volatile liquid that is easily soluble in water to form Hydrates. A geminal diol, also known as monohydrate, 2,2-dichloro-1,1-ethanediol, is formed in water.[1]
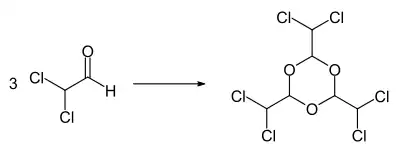
The compound decomposes when heated. In the presence of Lewis acids such as antimony trichloride, iron(III) chloride, aluminum trichloride, tin(IV) chloride or boron trifluoride, the trimer hexachloroparaldehyde (2,4,6-tris(dichloromethyl)-1,3,5-trioxane) can be obtained.[1] The trimer forms colourless crystals that melt at 131–132 °C. At the boiling point of 210–220 °C, dichloroacetaldehyde decomposes.[1]
Reduction with lithium aluminium hydride gives dichloroethanol.[2]
Uses
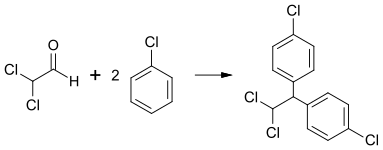
Dichloroacetaldehyde is used to produce other chemical compounds such as mitotane. Condensation with chlorobenzene yields p,p′-dichloro-1,1-diphenyl-2,2-dichloroethane, which was previously used as an insecticide:[1]
Synthesis
Dichloroacetaldehyde can be obtained by chlorinating acetaldehyde or paraldehyde. Hypochlorination of 1,2-dichloroethylene using chlorine and water produces pure dichloroacetaldehyde.[1][3]
References
- 1 2 3 4 5 Jira, R.; Kopp, E.; McKusick, B.C.; Röderer, G.; Bosch, A.; Fleischmann, G.: Chloroacetaldehydes in Ullmann’s Encyclopedia of Industrial Chemistry, 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, doi:10.1002/14356007.a06_527.pub2.
- ↑ Sroog, C. E.; Woodburn, H. M. (1952). "2,2-Dichloroethanol". Organic Syntheses. 32: 46. doi:10.15227/orgsyn.032.0046.
- ↑ NLM Hazardous Substances Data Bank entry for Dichloroacetaldehyde