 | |
| Names | |
|---|---|
| Preferred IUPAC name
4,5-Dichloro-2-octyl-1,2-thiazol-3(2H)-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.058.930 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H15Cl2NOS | |
| Molar mass | 282.23 |
| Appearance | white solid |
| Hazards | |
| GHS labelling: | |
   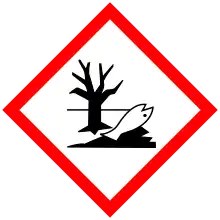 | |
| Danger | |
| H302, H312, H314, H317, H330, H331, H335, H410 | |
| P260, P261, P264, P270, P271, P272, P273, P280, P284, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P311, P312, P320, P321, P322, P330, P333+P313, P363, P391, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Dichlorooctylisothiazolinone, DCOIT or DCOI, is the organic compound with the formula SC(Cl)=C(Cl)C(O)NC7H15. It is a white solid that melts near room temperature. It is an isothiazolinone, a class of heterocyclic compounds used as biocides. DCOIT has attracted attention as an antifouling compound. It is a replacement for organotin compounds that have been largely banned for causing environmental damage. DCOIT however is itself controversial.[1]
Safety
Isothiazolinones are highly bioactive and have attracted scrutiny for causing contact dermatitis.[1]
References
- 1 2 Silva, Vânia; Silva, Cátia; Soares, Pedro; Garrido, E. Manuela; Borges, Fernanda; Garrido, Jorge (2020). "Isothiazolinone Biocides: Chemistry, Biological, and Toxicity Profiles". Molecules. 25 (4): 991. doi:10.3390/molecules25040991. PMC 7070760. PMID 32102175.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.