 | |
| Names | |
|---|---|
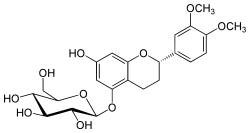
| IUPAC name
(2S)-2-(3,4-Dimethoxyphenyl)-7-hydroxy-3,4-dihydro-2H-1-benzopyran-5-yl β-D-glucopyranoside | |
| Systematic IUPAC name
(2S,3R,4S,5S,6R)-2-{[(2S)-2-(3,4-Dimethoxyphenyl)-7-hydroxy-3,4-dihydro-2H-1-benzopyran-5-yl]oxy}-6-(hydroxymethyl)oxane-3,4,5-triol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C23H28O10 | |
| Molar mass | 464.467 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Diffutin is a flavan, a type of flavonoid. It can be found in Canscora diffusa[1] and in Hoppea dichotoma.[2]
Metabolism
Diffutin is a glucoside of diffutidin.[1]
References
- 1 2 Shibnath Ghosal, Saini K. S. and Sinha B. N. (1983). "Diffutin, a new adaptogenic glucosyloxyflavan from Canscora diffusa". Journal of Chemical Research, Synopses (12).
- ↑ Ghosal, Shibnath; k. Jaiswal, Dinesh; k. Singh, Sushil; s. Srivastava, Radhey (1985). "Dichotosin and dichotosinin, two adaptogenic glucosyloxy flavans from Hoppea dichotoma". Phytochemistry. 24 (4): 831–833. doi:10.1016/S0031-9422(00)84903-1.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.