The diisopropylbenzenes (DIPB) are organic compounds with the formula C6H4(CH(CH3)2)2. Three isomers exist: 1,2- 1,3-, and 1,4-diisopropylbenzene. All are colorless liquids, immiscible in water, with similar boiling points. They are classified are aromatic hydrocarbons bearing a pair of isopropyl (CH(CH3)2) substituents.[1] DIPB has been referred to as "a common diluent" alongside hexane.[2]
| Diisopropylbenzenes | |||
| Systematic name | 1,2-Diisopropylbenzene | 1,3-Diisopropylbenzene | 1,4-Diisopropylbenzene |
| Common name | o-Diisopropylbenzene | m-Diisopropylbenzene | p-Diisopropylbenzene |
| Chemical structure | 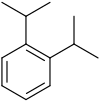 |
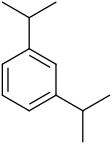 |
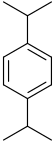 |
| CAS Number | 577-55-9 | 99-62-7 | 100-18-5 |
| PubChem | CID 11345 from PubChem | CID 7450 from PubChem | CID 7486 from PubChem |
| Chemical formula | C12H18 | ||
| Molar mass | 162.28 g/mol | ||
| State of matter | Liquid | ||
| Melting point[3] | −57 °C | −63 °C | −17 °C |
| Boiling point[3] | 205 °C | 203 °C | 210 °C |
| Solubility | Very slightly soluble in water[4] | 0.072 mg·l−1 in water (25 °C)[5] | Practically insoluble in water[6] |
Production and reactions
Diisopropylbenzenes typically arise by alkylation of benzene or isopropylbenzene with propylene:
- C6H6 + CH3CH=CH2 → C6H5CH(CH3)2
- C6H5CH(CH3)2 + CH3CH=CH2 → C6H4(CH(CH3)2)2
These alkylations are catalyzed by various Lewis acids, such as aluminium trichloride.
They can also be prepared and transformed by transalkylation reactions. In this way, triisopropylbenzenes are converted back to diisopropylbenzenes upon treatment with benzene or monoisopropylbenzene. As usual, these transformations are catalyzed by Lewis acids.[3]
- C6H4(CH(CH3)2)2 + C6H6 → 2 C6H5CH(CH3)2
The 1,3- and 1,4- isomers are mainly of interest as precursors to the respective dihydroxylbenzene derivatives, which exploits the Hock rearrangements. All three isomers form hydroperoxides, as is implicit in the Hock rearrangement, which are of interest as radical initiators for polymerization.[7]
See also
- Propofol, which is 1,3-DIPB with a hydroxyl group at position 2 (taken as position 1 in the propofol molecule)
References
- ↑ Vora BV, Kocal JA, Barger PT, Schmidt RJ, Johnson JA (2003). "Alkylation". Kirk-Othmer Encyclopedia of Chemical Technology. Vol. 2. John Wiley & Sons. doi:10.1002/0471238961.0112112508011313.a01.pub2. ISBN 9780471238966.
- ↑ Wang LY, Guo QJ, Lee MS (9 August 2018). "Recent advances in metal extraction improvement: Mixture systems consisting of ionic liquid and molecular extractant". Separation and Purification Technology (Review article). 210: 292–303. doi:10.1016/j.seppur.2018.08.016. S2CID 105020998.
- 1 2 3 Schmidt, Roland; Griesbaum, Karl; Behr, Arno; Biedenkapp, Dieter; Voges, Heinz-Werner; Garbe, Dorothea; Paetz, Christian; Collin, Gerd; Mayer, Dieter; Höke, Hartmut (2014). "Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. pp. 1–74. doi:10.1002/14356007.a13_227.pub3. ISBN 9783527306732.
- ↑ Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ↑ Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ↑ Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ↑ Schmiedel, Klaus W.; Decker, Daniel (2011). "Resorcinol". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a23_111.pub2. ISBN 9783527303854.
External links
 Media related to Diisopropylbenzenes at Wikimedia Commons
Media related to Diisopropylbenzenes at Wikimedia Commons