 | |
| Names | |
|---|---|
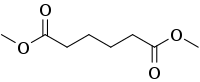
| Preferred IUPAC name
Dimethyl hexanedioate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.010.019 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H14O4 | |
| Molar mass | 174.196 g·mol−1 |
| Appearance | Colorless liquid[1] |
| Density | 1.06 g/cm3 (20 °C)[1] |
| Melting point | 10.3 °C (50.5 °F; 283.4 K)[1] |
| Boiling point | 227 °C (441 °F; 500 K)[1] |
| < 1 g/L[1] | |
| Viscosity | 2.5 cP @ 25°C |
| Hazards | |
| Flash point | 107 °C (225 °F; 380 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Dimethyl adipate is the organic compound with the formula (CH2CH2CO2CH3)2. It is a colorless oily liquid. Although the main commercial interest in adipates is related to the production of nylons, this diester is used as a plasticizer, a solvent for paint stripping and resins, and a pigment dispersant.[2][3]
Preparation
Dimethyl adipate is prepared by esterification of adipic acid with methanol. Less conventional routes include the hydroesterification of butadiene and the carbonylation of 1,4-dimethoxy-2-butene.[2]
It reacts with concentrated ammonia to give the diamide (CH2CH2C(O)NH2)2.
Toxicity
Esters of adipic acid exhibit low acute toxicities in animal models. The LD50 of this dimethyl ester is estimated at 1800 mg/kg (rat, i.p.).[2]
References
- 1 2 3 4 5 6 Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- 1 2 3 Musser, M. T. (2005). "Adipic Acid". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_269. ISBN 3527306730.
- ↑ "Dimethyl Adipate". chemicalland21.com.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.