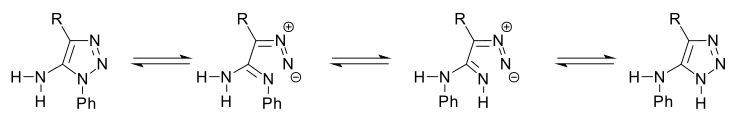
The Dimroth rearrangement is a rearrangement reaction taking place with certain 1,2,3-triazoles where endocyclic and exocyclic nitrogen atoms switch place.[1] This organic reaction was discovered in 1909 by Otto Dimroth.[2][3][4]
With R a phenyl group the reaction takes place in boiling pyridine for 24 hours.[5]
This type of triazole has an amino group in the 5 position. After ring-opening to a diazo intermediate, C-C bond rotation is possible with 1,3-migration of a proton.
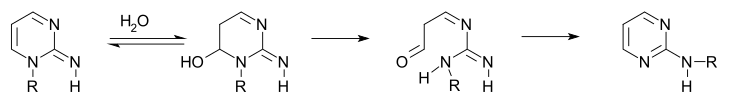
Certain 1-alkyl-2-iminopyrimidines also display this type of rearrangement.
In the first step is an addition reaction of water followed by ring-opening of the hemiaminal to the aminoaldehyde followed by ring closure.
A known drug example of the Dimroth rearrangement includes in the synthesis of Bemitradine [88133-11-3].
References
- ↑ Heterocyclic chemistry T.L. Gilchrist ISBN 0-582-01421-2
- ↑ Ueber intramolekulare Umlagerungen. Umlagerungen in der Reihe des 1, 2, 3-Triazols Justus Liebig's Annalen der Chemie Volume 364, Issue 2, Date: 1909, Pages: 183-226 Otto Dimroth doi:10.1002/jlac.19093640204
- ↑ Intramolekulare Umlagerung der 5-Amino-1,2,3-triazole Justus Liebig's Annalen der Chemie Volume 459, Issue 1, Date: 1927, Pages: 39-46 Otto Dimroth, Walter Michaelis doi:10.1002/jlac.19274590104
- ↑ Mittheilungen Ueber eine Synthese von Derivaten des 1.2.3-Triazols Otto Dimroth Berichte der deutschen chemischen Gesellschaft 1902 Volume 35, Issue 1 , Pages 1029 - 1038 doi:10.1002/cber.190203501171
- ↑ Organic Syntheses, Coll. Vol. 4, p.380 (1963); Vol. 37, p.26 (1957). http://orgsynth.org/orgsyn/pdfs/CV4P0380.pdf