 | |
| Names | |
|---|---|
| Preferred IUPAC name
Dipotassium cycloocatetraenediide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C8H8K2 | |
| Appearance | beige solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Dipotassium cyclooctatetraenide, sometimes abbreviated K2COT, is an organopotassium compound with the formula K2C8H8. It is a brown solid that is used as a precursor to cyclooctatetraenide complexes, such as uranocene (U(C8H8)2). Analogs of K2C8H8 are known with ring substituents, with different alkali metals, and with various complexants.
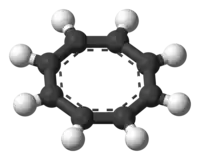
Preparation and structure
Potassium cyclooctatetraenide is formed by the reaction of cyclooctatetraene with potassium metal:
- 2 K + C8H8 → K2C8H8
The reaction entails 2-electron reduction of the polyene and is accompanied by a color change from colorless to brown.[1]
The structure of K2(diglyme)C8H8 has been characterized by X-ray crystallography of the derivatives with diglyme complexed to the potassium cations. The C8H8 unit is planar with an average C-C distance of 1.40 A.[2]
References
- ↑ A. L Wayda (1990). "Cyclooctatetraene Lanthanide Complexes. Lu(C8 H8 )Cl(THF) and Lu(C8 H8 )[o -C6 H4 CH2 N(CH3 )2 ](THF)". Cyclooctatetraene Lanthanide Complexes. Lu(C8H8Cl(thf) and Lu(C8H8)[o-C6H4CH2N(CH3)2(thf). Inorganic Syntheses. Vol. 27. pp. 150–154. doi:10.1002/9780470132586.ch28. ISBN 9780470132586.
- ↑ J. H. Noordik, T. E. M. van den Hark, J. J. Mooij and A. A. K. Klaassen (1974). "Dipotassium(I) cyclooctatetraenide-1-methoxy-2-(2-methoxyethoxy)ethane". Acta Crystallogr B. 30 (3): 833–835. doi:10.1107/S0567740874003840.
{{cite journal}}: CS1 maint: multiple names: authors list (link)