 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Disulfur dichloride | |||
| Systematic IUPAC name
Dichlorodisulfane | |||
Other names
| |||
| Identifiers | |||
| |||
3D model (JSmol) |
|||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.030.021 | ||
| EC Number |
| ||
| MeSH | Sulfur+monochloride | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 3390 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
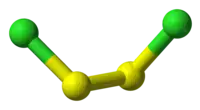
| S2Cl2 | |||
| Molar mass | 135.02 g·mol−1 | ||
| Appearance | Light-amber to yellow-red, oily liquid[1] | ||
| Odor | pungent, nauseating, irritating[1] | ||
| Density | 1.688 g/cm3 | ||
| Melting point | −80 °C (−112 °F; 193 K) | ||
| Boiling point | 137.1 °C (278.8 °F; 410.2 K) | ||
| Decomposes, with loss of HCl | |||
| Solubility | Soluble in ethanol, benzene, ether, THF, chloroform, CCl4[2] | ||
| Vapor pressure | 7 mmHg (20 °C)[1] | ||
| −62.2·10−6 cm3/mol | |||
Refractive index (nD) |
1.658 | ||
| Structure | |||
| C2 | |||
| 2 at sulfur atoms | |||
| gauche | |||
| 1.60 D[2] | |||
| Hazards | |||
| GHS labelling: | |||
    | |||
| Danger | |||
| H301, H314, H332, H400 | |||
| P260, P261, P264, P270, P271, P273, P280, P301+P310, P301+P330+P331, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P330, P363, P391, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 118.5 °C (245.3 °F; 391.6 K) | ||
| 234 °C (453 °F; 507 K) | |||
| Lethal dose or concentration (LD, LC): | |||
LCLo (lowest published) |
150 ppm (mouse, 1 min) (1 ppm = 5.52 mg/m3)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 1 ppm (5.52 mg/m3)[1] | ||
REL (Recommended) |
C 1 ppm (5.52 mg/m3)[1] | ||
IDLH (Immediate danger) |
5 ppm[1] (1 ppm = 5.52 mg/m3) | ||
| Safety data sheet (SDS) | ICSC 0958 | ||
| Related compounds | |||
Related sulfur chlorides/oxychlorides |
|||
Related compounds |
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Disulfur dichloride (or disulphur dichloride by the British English spelling) is the inorganic compound of sulfur and chlorine with the formula S2Cl2.[4][5][6][7] It is an amber oily liquid.
Sometimes, this compound is incorrectly named sulfur monochloride (or sulphur monochloride by the British English spelling), the name implied by its empirical formula SCl.
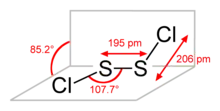
S2Cl2 has the structure implied by the formula Cl−S−S−Cl, wherein the dihedral angle between the Cla−S−S and S−S−Clb planes is 85.2°. This structure is referred to as gauche, and is akin to that for H2O2. A rare isomer of S2Cl2 is S=SCl2 (thiothionyl chloride); this isomer forms transiently when S2Cl2 is exposed to UV-radiation (see thiosulfoxides).
Synthesis, basic properties, reactions
Disulfur dichloride is a yellow liquid that fumes in moist air due to reaction with water:
- 16 S2Cl2 + 16 H2O → 8 SO2 + 32 HCl + 3 S8
It is produced by partial chlorination of elemental sulfur. The reaction proceeds at usable rates at room temperature. In the laboratory, chlorine gas is led into a flask containing elemental sulfur. As disulfur dichloride is formed, the contents become a golden yellow liquid:[8]
- S8 + 4 Cl2 → 4 S2Cl2, ΔH = −58.2 kJ/mol
Excess chlorine produces sulfur dichloride, which causes the liquid to become less yellow and more orange-red:
- S2Cl2 + Cl2 ⇌ 2 SCl2, ΔH = −40.6 kJ/mol
The reaction is reversible, and upon standing, SCl2 releases chlorine to revert to the disulfur dichloride. Disulfur dichloride has the ability to dissolve large quantities of sulfur, which reflects in part the formation of polysulfanes:
- 8 S2Cl2 + n S8 → 8 Sn+2Cl2
Disulfur dichloride can be purified by distillation from excess elemental sulfur.
S2Cl2 also arises from the chlorination of CS2 as in the synthesis of thiophosgene or carbon tetrachloride.
Reactions
S2Cl2 hydrolyzes to sulfur dioxide and elemental sulfur. When treated with hydrogen sulfide, polysulfanes are formed as indicated in the following idealized formula:
- 2 H2S + S2Cl2 → H2S4 + 2 HCl
It reacts with ammonia to give heptasulfur imide (S7NH) and related S−N rings S8−n(NH)n (n = 2, 3).
Applications
S2Cl2 has been used to introduce C−S bonds. In the presence of aluminium chloride (AlCl3), S2Cl2 reacts with benzene to give diphenyl sulfide:
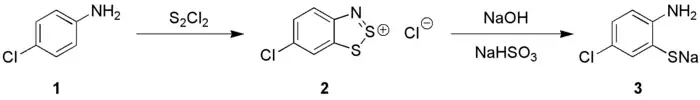
Anilines (1) react with S2Cl2 in the presence of NaOH to give 1,2,3-benzodithiazolium chloride (2) (Herz reaction) which can be transformed into ortho-aminothiophenolates (3), these species are precursors to thioindigo dyes.
It is also used to prepare mustard gas via ethylene at 60 °C (the Levinstein process):
- 8 S2Cl2 + 16 H2C=CH2 → 8 (ClCH2CH2)2S + S8
Other uses of S2Cl2 include the manufacture of sulfur dyes, insecticides, and synthetic rubbers. It is also used in cold vulcanization of rubbers, as a polymerization catalyst for vegetable oils and for hardening soft woods.[9]
Safety and regulation
S2Cl2 can be used to produce bis(2-chloroethyl)sulfide S(CH2CH2Cl)2, known as the mustard gas:[9]
Consequently, it is listed in Schedule 3 of the Chemical Weapons Convention. Facilities that produce and/or process and/or consume scheduled chemicals may be subject to control, reporting mechanisms and inspection by the Organisation for the Prohibition of Chemical Weapons.
References
- 1 2 3 4 5 6 NIOSH Pocket Guide to Chemical Hazards. "#0578". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- ↑ "Sulfur monochloride". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ↑ Holleman, A. F.; Wiberg, E. Inorganic Chemistry Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ↑ Hartman, W. W.; Smith, L. A.; Dickey, J. B. (1934). "Diphenylsulfide". Organic Syntheses. 14: 36.; Collective Volume, vol. 2, p. 242
- ↑ R. J. Cremlyn An Introduction to Organosulfur Chemistry John Wiley and Sons: Chichester (1996). ISBN 0-471-95512-4
- ↑ Garcia-Valverde M., Torroba T. (2006). "Heterocyclic chemistry of sulfur chlorides – Fast ways to complex heterocycles". European Journal of Organic Chemistry. 2006 (4): 849–861. doi:10.1002/ejoc.200500786.
- ↑ F. Fehér "Dichlorodisulfane" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 371.
- 1 2 Lauss, Hans-Dietrich; Steffens, Wilfried (2000). "Sulfur Halides". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a25_623. ISBN 3527306730.