 | |
| Clinical data | |
|---|---|
| Trade names | Urece |
| Other names | FYU-981 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
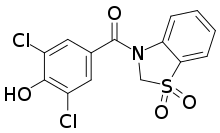
| Formula | C14H9Cl2NO4S |
| Molar mass | 358.19 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Dotinurad (Urece) is a drug for the treatment of gout and hyperuricemia.[1][2] It was developed by Fuji Yakuhin and approved for use in Japan in 2020.[2][3] The drug is continuing clinical trials by Fortress Biotech and regulatory evaluation for approval in North America and Europe.[3][4]
Dotinurad acts as a selective urate reabsorption inhibitor that has uric acid lowering activity.[5][6]
References
- ↑ Kuriyama S (March 2020). "Dotinurad: a novel selective urate reabsorption inhibitor as a future therapeutic option for hyperuricemia". Clinical and Experimental Nephrology. 24 (Suppl 1): 1–5. doi:10.1007/s10157-019-01811-9. PMC 7066308. PMID 31754883.
- 1 2 "List of Approved Products" (PDF). Pharmaceuticals and Medical Devices Agency.
- 1 2 "Fortress takes on dotinurad in USA and Europe". November 5, 2020. Retrieved June 23, 2021.
- ↑ "Fortress Biotech Announces Exclusive License Agreement With Fuji Yakuhin to Develop Dotinurad in North America and Europe". May 10, 2021. Retrieved June 23, 2021.
- ↑ Taniguchi T, Ashizawa N, Matsumoto K, Saito R, Motoki K, Sakai M, et al. (October 2019). "Pharmacological Evaluation of Dotinurad, a Selective Urate Reabsorption Inhibitor". The Journal of Pharmacology and Experimental Therapeutics. 371 (1): 162–170. doi:10.1124/jpet.119.259341. PMID 31371478. S2CID 199382932.
- ↑ Uda J, Kobashi S, Miyata S, Ashizawa N, Matsumoto K, Iwanaga T (October 2020). "Discovery of Dotinurad (FYU-981), a New Phenol Derivative with Highly Potent Uric Acid Lowering Activity". ACS Medicinal Chemistry Letters. 11 (10): 2017–2023. doi:10.1021/acsmedchemlett.0c00176. PMC 7549256. PMID 33062187.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.