 | |
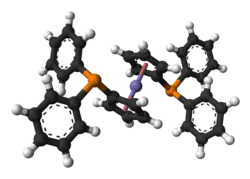 | |
| Names | |
|---|---|
| Preferred IUPAC name
(Ferrocene-1,1′-diyl)bis(diphenylphosphane) | |
| Other names
1,1′-Bis(diphenylphosphino)ferrocene, 1,1′-ferrocenediylbis(diphenylphosphine), Dppf, 1,1′-ferrocenebis(diphenylphosphine), 1,1′-bis(diphenylphosphanyl)ferrocene | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.167.773 |
| EC Number |
|
| 24075 | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C34H28FeP2 | |
| Molar mass | 554.391 |
| Melting point | 181 to 183 °C (358 to 361 °F; 454 to 456 K) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Toxic |
| GHS labelling: | |
  | |
| Warning | |
| H302, H312, H315, H319, H332 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
1,1′-Bis(diphenylphosphino)ferrocene, commonly abbreviated dppf, is an organophosphorus compound commonly used as a ligand in homogeneous catalysis. It contains a ferrocene moiety in its backbone, and is related to other bridged diphosphines such as 1,2-bis(diphenylphosphino)ethane (dppe).
Preparation
This compound is commercially available. It may be prepared by treating dilithioferrocene with chlorodiphenylphosphine:[1]
- Fe(C5H4Li)2 + 2 ClPPh2 → Fe(C5H4PPh2)2 + 2 LiCl
The dilithiation of ferrocene is easily achieved with n-butyllithium in the presence of TMEDA. Many related ligands can be made in this way. The Fe center is typically not involved in the behavior of the ligand.
Reactions
Dppf readily forms metal complexes.[2] The palladium derivative, (dppf)PdCl2, which is popular for palladium-catalyzed coupling reactions, is prepared by treating dppf with the acetonitrile or benzonitrile adducts of palladium dichloride:[2] Substitution of the phenyl substituents in dppf leads to derivatives with modified donor-acceptor properties at the phosphorus atoms.[3]
- dppf + PdCl2(RCN)2 → (dppf)PdCl2 + 2 RCN (RCN = acetonitrile or benzonitrile)
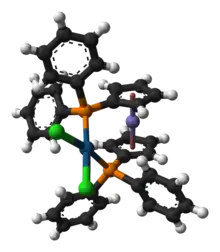
Another example of dppf in homogeneous catalysis is provided by the air- and moisture-stable Ni(II) precatalyst [(dppf)Ni(cinnamyl)Cl. It promotes Suzuki-Miyuara cross-coupling of heteroaryl boronic acids with nitrogen- and sulfur-containing heteroaryl halides.[4]
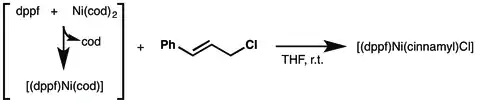
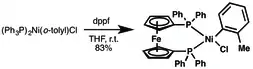
Another dppf-based catalyst is (dppf)Ni(o-tolyl)Cl, can be prepared from ligand exchange with (PPh3)2Ni(o-tolyl)Cl. It promotes the amination of aryl chlorides, sulfamates, mesylates, and triflates.[5]
See also
- Diphosphines
- borrowing hydrogen catalysis
References
- ↑ Ian R. Butler (2010). "3.15 The Use of Organolithium Reagents in the Preparation of Ferrocene Derivatives" (Google Books excerpt). In J. Derek Woollins (ed.). Inorganic Experiments. John Wiley & Sons. pp. 175–179. ISBN 978-3-527-32472-9.
- 1 2 Nataro, Chip; Fosbenner, Stephanie M. (2009). "Synthesis and Characterization of Transition-Metal Complexes Containing 1,1′-Bis(diphenylphosphino)ferrocene". J. Chem. Educ. 86 (12): 1412. Bibcode:2009JChEd..86.1412N. doi:10.1021/ed086p1412.
- ↑ Dey, Subhayan; Pietschnig, Rudolf (2021). "Chemistry of sterically demanding dppf-analogs". Coord. Chem. Rev. 437: 213850. doi:10.1016/j.ccr.2021.213850.
- ↑ Ge, Shaozhong; Hartwig, John F. (2012-12-14). "Highly Reactive, Single-Component Nickel Catalyst Precursor for Suzuki–Miyuara Cross-Coupling of Heteroaryl Boronic Acids with Heteroaryl Halides". Angewandte Chemie International Edition. 51 (51): 12837–12841. doi:10.1002/anie.201207428. PMC 3613336. PMID 23136047.
- ↑ Park, Nathaniel H.; Teverovskiy, Georgiy; Buchwald, Stephen L. (2014-01-03). "Development of an Air-Stable Nickel Precatalyst for the Amination of Aryl Chlorides, Sulfamates, Mesylates, and Triflates". Organic Letters. 16 (1): 220–223. doi:10.1021/ol403209k. PMC 3926134. PMID 24283652.