 | |
 | |
| Names | |
|---|---|
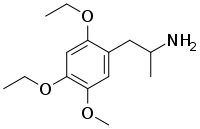
| Preferred IUPAC name
1-(2,4-Diethoxy-5-methoxyphenyl)propan-2-amine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H23NO3 | |
| Molar mass | 253.342 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
EEM (2,4-diethoxy-5-methoxyamphetamine) is a lesser-known substituted amphetamine. It is a diethoxy-methoxy analog of trimethoxyamphetamine (TMA-2). EEM was first synthesized by Alexander Shulgin. In his book PiHKAL, both the dosage and duration are unknown.[1] EEM produces few to no effects. Very little data exists about the pharmacological properties, metabolism, and toxicity of EEM.
See also
References
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.