| Echinococcus multilocularis | |
|---|---|
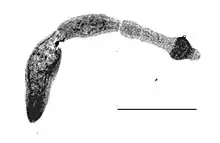 | |
| Echinococcus multilocularis isolated from a fox | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Platyhelminthes |
| Class: | Cestoda |
| Order: | Cyclophyllidea |
| Family: | Taeniidae |
| Genus: | Echinococcus |
| Species: | E. multilocularis |
| Binomial name | |
| Echinococcus multilocularis Leuckart, 1863 | |
| Synonyms | |
Echinococcus multilocularis, the fox tapeworm, is a small cyclophyllid tapeworm found extensively in the northern hemisphere. E. multilocularis, along with other members of the Echinococcus genus (especially E. granulosus), produce diseases known as echinococcosis. Unlike E. granulosus, E. multilocularis produces many small cysts (also referred to as locules) that spread throughout the internal organs of the infected animal. The resultant disease is called Alveolar echinococcosis, and is caused by ingesting the eggs of E. multilocularis.
The parasite is commonly maintained in a wildlife life cycle involving two mammalian hosts. Wild canids, dogs, and less commonly cats act as definitive hosts, harbouring the adult stage of the tape worm. Voles are the intermediate hosts.[1] Ingestion of a rodent containing alveolar hydatid cysts by a wild canid can result in a heavy infestation of tapeworms.
Signs and symptoms
Human alveolar echinococcosis is characterized by a lengthy incubation period of 5 to 15 years in immunocompetent individuals. The progression of disease is potentiated in immunocompromised patients. Following the ingestion of the eggs of E. multilocularis, the metacestode (larval) stage of the parasite typically embeds in the liver. As the disease progresses, the larval stage proliferates exogenously within the tissue, behaving similar to hepatic neoplasia. Patients with human alveolar echinococcosis typically present with headache, nausea, vomiting, abdominal pain. Jaundice is rare,[2] but hepatomegaly is a common physical finding.
Life cycle
The life cycle of E. multilocularis involves a primary or definitive host and a secondary or intermediate host, each harboring different life stages of the parasite.
Foxes, coyotes, domestic dogs, and other canids are the definitive hosts for the adult stage of the parasite. Cats may also be involved.[3] The head of the tapeworm attaches to the intestinal mucosa by hooks and suckers. It then produces hundreds of microscopic eggs, which are dispersed through the feces.[4]
Voles are the intermediate host.[1] Beavers may also be affected; and reintroduction of beavers from affected areas is a cause for concern.[5] Eggs ingested by rodents develop in the liver, lungs and other organs to form multilocular cysts. The life cycle is completed after a fox or canine consumes a rodent infected with cysts. Larvae within the cyst develop into adult tapeworms in the intestinal tract of the definitive host.[4]
Humans can become an aberrant intermediate host by accidentally ingesting eggs of E. multilocularis when handling infected animals or consuming contaminated food, vegetable, and water. Except in rare cases where infected humans are eaten by canines, humans are a dead-end or incidental host (an aberrant intermediate host that does not allow transmission to the definitive host) for E. multilocularis.
- Summary of the life cycle
- adult worm present in intestine of definitive host
- eggs passed in feces, ingested by humans or intermediate host
- onchosphere penetrates intestinal wall, carried via blood vessels to lodge in organs
- hydatid cysts develop in liver, lungs, brain, heart
- protoscolices (hydatid sand) ingested by definitive host
- ingested protoscolices attach to small intestine and develops into adult worm
Morphology

The adult parasite is a small tapeworm that is 3-6 mm long, and lives in the small intestine of canines. The segmented worm contains a scolex with suckers and hooks that enable attachment to the mucosal wall, since tapeworms do not have a digestive tract. A short neck connects the head to three proglottids, the body segment of the worm which contains the eggs to be excreted in the feces.[6]
Diagnosis
Serological and imaging tests are commonly used to diagnose this disease. Since the serological tests for alveolar echinococcosis only indicate exposure to the parasite and not ongoing infection, visualization of the parasitic mass is required to confirm the diagnosis. Frequently used serological tests include antibody tests, ELISA and indirect hemaglutination (IHA). An intradermal allergic reaction test (Casoni test) has also been used to diagnose patients. Imaging tests include: X-rays, CT scans, MRI, and ultrasound.[2] Histology is another important diagnostic tool to use. In the past, the PAS staining technique has proven beneficial in helping determine if infections are caused by Echinococcus multiocularis. More recently, a new immunohistochemistry technique that uses a monoclonal antibody (Em2G11) has provided professionals with more accuracy and precision in diagnosing Echinococcus multiocularis and distinguishing it from other species like Echinococcus granulosus.[7]
Disease staging
Alveolar echinococcosis (AE) is a highly lethal helminthic disease in humans, caused by the larval form of the parasitic tapeworm E. multilocularis. The disease represents a serious public threat in China, Siberia, and central Europe. However, since the 1990s, the prevalence of the disease seems to be increasing in Europe, not only in the historically endemic areas but its neighboring regions.[8] AE primarily affects the liver by inducing a hepatic disorder similar to liver cancer, therefore becoming extremely dangerous and difficult to diagnose. If the infection metastasizes, it may spread to any other organ and could be lethal if not treated. The most common treatment for AE is to surgically remove the parasite. Since it is difficult and not always possible to remove the entire parasite, medicine such as Albendazole is utilized to keep the cyst from growing back.[4]
Guided by the Tumor-Node-Metastasis (TNM) system of liver cancer, the European Network for Concerted Surveillance of Alveolar Echinococcosis and the World Health Organization Informal Working Group on Echinococcosis, a clinical classification system has been proposed. This classification system has been designated as the "PNM" system (P = parasitic mass, N = involvement of neighboring organs, M = metastasis). The system was developed by a retrospective analysis of records from 97 patients treated in France and Germany (2 treatment centers). Amongst other characteristics, the system takes into consideration the localization of the parasite in the liver, the extent of lesion involvement, regional involvement, and metastasis.[9]
Treatment
If no specific therapy is initiated, in 94% of patients the disease is fatal within 10–20 years following diagnosis.[10]
- Currently, benzimidazoles (such as albendazole) are used to treat AE: only halt their proliferation and do not actually kill the parasites, side effects such as liver damage
- 2-ME2, a natural metabolite of estradiol, is tested with some results in vitro: decreased transcription of 14-3-3-pro-tumorogenic zeta-isoform, causes damage to germinal layer but does not kill parasite in vivo
- Treatment with a combination of albendazole/2-ME2 showed best results in reducing parasite burden[11]
- Despite the improvements in the chemotherapy of echinococcosis with benzimidazole derivatives, complete elimination of the parasitic mass cannot be achieved in most infected patients, although studies indicate that long-term treatment with mebendazole typically increases the survival rate.[12].
Epidemiology
The incidence of human infestation with E. multilocularis and disease is increasing in urban areas, as wild foxes (an important reservoir species of the sylvatic cycle) are migrating to urban and suburban areas and gaining closer contact with human populations.[4] Also, restocking fox enclosures for fox hunting with infected animals spreads the disease.[14] Children, health care workers and domestic animals are at risk of ingesting the eggs after coming into contact with the feces of infected wild foxes. Even with the improvement of health in developed/industrialized countries, the prevalence of alveolar echinococcosis (AE) did not decrease.[4] On the contrary, incidents of AE have now also been registered in eastern European countries and sporadic incidences in other European countries.[4]
The disease has extended its range in Europe in the last few decades.[15] Still the infection is fairly rare. Between 1982 and 2000 a total of 559 cases were reported throughout Europe.[16]
Recent findings indicate that E. multilocularis is likely expanding its range in the central region of the United States and Canada and that invasions of European strains might have occurred; the endemic presence of the parasite in urban areas and a total of 16 human cases in Alberta, Canada have been reported.[13][17] The first human case in Alberta was diagnosed in 2013. In one case, the infection caused a grapefruit sized tumour in the lung, kidney and diaphragm, which had to be surgically removed.[17] Because of the significant difficulty in diagnosising an infection caused by Echinococcus multiocularis, it is suspected that this parasite is often misdiagnosed. Several studies that have been done since the 1950s in North America indicate a higher prevalence of Echinococcus multiocularis than previously thought. However, due to limited knowledge about the parasite, it is often not monitored in wild or domestic animals; this monitoring will become crucial to the control of the parasite in the future as it continues to spread.[13]
See also
References
- 1 2 3 4 5 Nakao, Minoru; Yanagida, Tetsuya; Okamoto, Munehiro; Knapp, Jenny; Nkouawa, Agathe; Sako, Yasuhito; Ito, Akira (2010). "State-of-the-art Echinococcus and Taenia: Phylogenetic taxonomy of human-pathogenic tapeworms and its application to molecular diagnosis". Infection, Genetics and Evolution. Elsevier. 10 (4): 444–452. doi:10.1016/j.meegid.2010.01.011. ISSN 1567-1348. PMID 20132907.
- 1 2 Kayacan SM, Vatansever S, Temiz S, et al. (January 2008). "Alveolar echinococcosis localized in the liver, lung and brain". Chin. Med. J. 121 (1): 90–2. doi:10.1097/00029330-200801010-00018. PMID 18208675.
- ↑ Knapp, Jenny; Combes, Benoît; Umhang, Gérald; Aknouche, Soufiane; Millon, Laurence (2016). "Could the domestic cat play a significant role in the transmission of Echinococcus multilocularis? A study based on qPCR analysis of cat feces in a rural area in France". Parasite. 23. 42. doi:10.1051/parasite/2016052. ISSN 1776-1042. PMC 5782850. PMID 27739398.
- 1 2 3 4 5 6 Echinococcosis at eMedicine
- ↑ Kosmider, Rowena; Gale, Paul; Paterson, Andy; Voas, Andrew; Mount, Louise; Roberts, Helen (June 2012). What is the risk of introducing Echinococcus multilocularis to the UK wildlife population by importing European beavers which subsequently escape or are released? Qualitative Risk Assessment (PDF) (Report). Department for Environment, Food and Rural Affairs. Archived from the original (PDF) on 2014-05-07. Retrieved 6 June 2017 – via UK Government Archive.
- ↑ John, David T.; William Petri, William A.; Markell, Edward K.; Voge, Marietta (January 2006). "7: The Cestodes: Echinococcus granulosus, E. multiloularis and E. vogeli (Hyatid Disease)". Markell and Voge's Medical Parasitology (9th ed.). Elsevier Health Sciences. pp. 224–231. ISBN 0-7216-4793-6.
- ↑ Barth TF, Hermann TS, Tappe D, et al. (2012). "Sensitive and Specific Immunohistochemical Diagnosis of Human Alveolar Echinococcosis with the Monoclonal Antibody Em2G11". PLOS Neglected Tropical Diseases. 6 (10). e1877. doi:10.1371/journal.pntd.0001877. PMC 3493387. PMID 23145198.
- ↑ Torgerson PR, Keller K, Magnotta M, Ragland N (2010). "The global burden of alveolar echinococcosis". PLOS Neglected Tropical Diseases. 4 (6). e722. doi:10.1371/journal.pntd.0000722. PMC 2889826. PMID 20582310.
- ↑ Kern P, Wen H, Sato N, et al. (2006). "WHO classification of alveolar echinococcosis: principles and application". Parasitol. Int. 55 (Suppl): S283–7. doi:10.1016/j.parint.2005.11.041. PMID 16343985.
- ↑ Jura H, Bader A, Frosch M (May 1998). "In vitro activities of benzimidazoles against Echinococcus multilocularis metacestodes". Antimicrob. Agents Chemother. 42 (5): 1052–6. doi:10.1128/AAC.42.5.1052. PMC 105743. PMID 9593125.
- ↑ Spicher M, Naguleswaran A, Ortega-Mora LM, Müller J, Gottstein B, Hemphill A (March 2008). "In vitro and in vivo effects of 2-methoxyestradiol, either alone or combined with albendazole, against Echinococcus metacestodes". Exp. Parasitol. 119 (4): 475–82. doi:10.1016/j.exppara.2008.02.012. PMID 18442817.
- ↑ Hemphill, Andrew; Stadelmann, Britta; Rufener, Reto; Spiliotis, Markus; Boubaker, Ghalia; Muller, Joachim; Muller, Norbert; Gorgas, Daniela; Gottstein, Bruno (2014). "Treatment of Echinococcosis: Albendazole and Mebendazole- what else?". Parasite. 21. 70. doi:10.1051/parasite/2014073. PMC 4271654. PMID 25526545.
- 1 2 3 Massolo, Alessandro; Liccioli, Stefano; Budke, Christine; Klein, Claudia (2014). "Echinococcus multilocularis in North America: the great unknown". Parasite. 21. 73. doi:10.1051/parasite/2014069. ISSN 1776-1042. PMC 4273702. PMID 25531581.
- ↑ Drisdelle, Rosemary (2010). Parasites: Tales of Humanity's Most Unwelcome Guests. University of California Press. p. 96. ISBN 978-0-520-25938-6.
- ↑ Sréter T, Széll Z, Sréter-Lancz Z, Varga I (July 2004). "Echinococcus multilocularis in Northern Hungary". Emerging Infect. Dis. 10 (7): 1344–6. doi:10.3201/eid1007.031027. PMC 3323332. PMID 15338552.
- ↑ Kern P, Bardonnet K, Renner E, et al. (March 2003). "European echinococcosis registry: human alveolar echinococcosis, Europe, 1982–2000". Emerging Infect. Dis. 9 (3): 343–9. doi:10.3201/eid0903.020341. PMC 2958541. PMID 12643830.
- 1 2 Fournier, Ariel (Jan 23, 2020). "For this Alberta woman, the good news was she had contracted a rare, deadly parasite". CBC News.
External links
- Echinococcus multilocularis
- "Echinococcus multilocularis". NCBI Taxonomy Browser. 6211.