A molecular electron-reservoir complex is one of a class of redox-active systems which can store and transfer electrons stoichiometrically or catalytically without decomposition. The concept of electron-reservoir complexes was introduced by the work of French chemist, Didier Astruc.[1][2] From Astruc's discoveries, a whole family of thermally stable, neutral, 19-electron iron(I) organometallic complexes were isolated and characterized, and found to have applications in redox catalysis and electrocatalysis.[3][4] The following page is a reflection of the prototypal electron-reservoir complexes discovered by Didier Astruc.
Synthesis
The parent complex, C5H5FeC6H6, is observed to undergo decomplexation and dimerization, whereas analogues containing six alkyl groups on the benzene ring exhibit stability in many solvents and remain catalytically active (written as η5-CpFe-η6-arene).[2]
Syntheses using ferrocene
In the process of ligand exchange, one of the cyclopentadienyl rings of ferrocene is replaced by an arene group.[5] This has garnered significant interest as it provides a simple means to form complexes of arenes with the CpFe+ units. The reaction of ferrocene and an aryl alkyl group is carried out at 70–90 °C for 1–16 hours in the arene as the solvent (Figure 1). Aluminum chloride, AlCl3, is a Lewis acid which prompts the reaction, and aluminum metal is added to inhibit oxidation of ferrocene to ferrocenium. Monoelectronic reduction of the FeII 18-electron monocation with Na/Hg in THF at ambient temperature yields the 19-electron complex as green-black crystals.
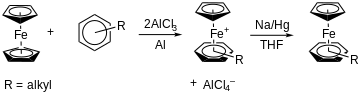
Electron donating groups on the arene increase the yield.[6] Additionally, alkyl groups which induce steric hindrance (e.g., Me, Et, etc.) will increase the yield.[7]
Complexation of arenes to iron
A known type of complexation is the Fischer-Hafner synthesis,[8] which treats transition metal chlorides with arenes in the presence of aluminum metal and aluminum chloride. The synthesis of bis(arene) iron dications is an example of this type, where a mixture of iron dichloride, FeCl2, alkylbenzenes, and AlCl3 is refluxed in the arene as the solvent (Figure 2).
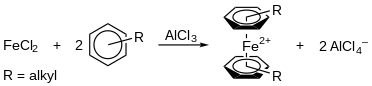
Although AlCl3 is almost always used to induce ligand exchange reactions, other efficient Lewis acids are AlBr3, GaCl3, ZrCl4, and HfCl4.[5]
Structure and bonding
Cyclopentadienyl iron(arene) complexes
The electronic spectra of CpFe+(arene) complexes have been compared to those of ferrocene and bis(arene) iron dications.[5] Three spin allowed transitions are observed at 22,200, 26,200 and 31,900 cm–1 and two bands at 38,200 and 41,800 cm–1 are attributed to π→π* benzene transitions. The ligand field parameters (cm–1) for the CpFe+ (arene) complexes are Δ1 = 8,500, Δ2 = 21,900, B = 320 (comparatively for ferrocene, Δ1 = 7,200, Δ2 = 22,000, B = 390). Therefore, the values of the electronic repulsion parameters B indicate large covalency between the metal–ligand bonds. In general, 19-electron complexes are thermally stable only when the arene ligand is peralkylated; steric bulk also stabilizes the complex.
The X-ray crystal structure for the CpFe(I)-C6Me6 complex exhibits alternating sandwich positions in the crystal packing.[9] Detailed EPR studies of the Fe(I) sandwiches in frozen solution, in the solid state, and in diamagnetic matrices show dynamic rhombic distortion, high degree of covalency, and spin-lattice relaxation.[9]
Bis(arene) iron dications
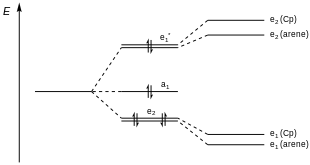
Bis(arene) iron dications and their electronic configuration bear resemblance to their bis(arene) chromium counterparts.[5] These compounds exhibit a doubly degenerate bonding e2 level that is high in metal character, while the nonbonding a1 orbital is nearly a pure dz2 metal orbital (Figure 3).
When one electron is added to the dication, (C6Me6)2Fe2+, a stable 19-electron complex is formed, a compound stabilized only when the arene is endowed with multiple methyl groups.[10] Remarkably, a nearly stable complex arises when a second extra electron is added, with the case of C6Me6 being particularly noteworthy (Figure 4). Although the effective atomic number (EAN) rule states that thermodynamically stable transition metal complexes contain 18 valence electrons, there are situations found in these organoiron systems where it can be breached, given that the anti-bonding doubly degenerate e1g level harbors a high metal character and is located at relatively low energy. Three isostructural complexes (C6Me6)2Fen+, where n ranges from 0 to 2, have been isolated and their structures are shown in Figure 4.
2Fe_Complexes.svg.png.webp)
Reactions and functionalizations of the coordinated arene
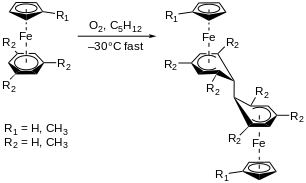
In 1979, Didier et al. reported the activation of the arene group in η5-C5H5Fe-η6-C6(CH3)6 by dioxygen, O2, through an electron transfer mechanism to form the superoxide radical anion, O2–•.[11] In this paper, two unique reactions of O2 are reported: hydrogen atom abstraction by O2 from a methyl group on the arene ring (Figure 5), and the O2-induced dimerization of C5H5Fe-arene when the arene group bears less than six methyl groups.
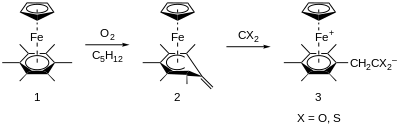
The green η5-C5H5Fe-η6-C6(CH3)6 complex reacts readily when in contact with air (25°C) in pentane to afford complex 2 and water (Figure 5). Characterization by mass spectrometry, nuclear magnetic resonance, and X-ray crystallography revealed that the structure of 2 is best described as a cyclohexadienyl ligand coordinated in a pentahapto fashion to η5-C5H5Fe and bearing an exocyclic double bond (Figure 5). Complex 2 has shown to be a good model for intermediates in benzylic activation processes when reacting with carbon dioxide, CO2, and carbon disulfide, CS2 (Figure 5, right-side).
Dioxygen induces dimerization for complexes shown in Figure 6. The O2-induced dimerization follows a radical mechanism, whereas H-atom abstraction in Figure 5 is mainly ionic.
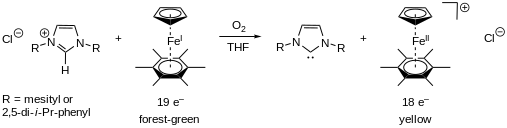
Other useful electron-transfer reactions of η5-C5H5Fe-η6-C6(CH3)6 is the deprotonation of imidazolium salts, generating N-heterocyclic (NHC) carbenes.[12] Arduengo demonstrated that deprotonating these salts produces NHCs that are isolable and relatively stable, provided that the heterocycle nitrogen atoms have appropriate substituents.[13] Reacting a stoichiometric amount of CpFe-C6(CH3)6 in THF in the presence of air with imidazolium salts, quickly results in the soluble carbenes, which are visible by the color change from deep-green to yellow (Figure 7).[12] The carbenes generated in this form were characterized by 1H and 13C NMR.
Further reading
In 2014, Chang et al. reported the synthesis and properties of bis(formazanate) zinc complexes.[14] These complexes exhibit reversible redox-chemistry, with the ligands serving as electron reservoirs. As a result, these complexes can be synthesized in three redox states, in which the formazanate ligands are reduced to "metallaverdazyl" radicals. The complexes were fully characterized using various methods, including single-crystal X-ray crystallography, spectroscopic techniques, and DFT calculations.
A decade prior, Venkatesan et al. investigated a series of electron-rich manganese(I) half-sandwich complexes for applications as molecular batteries. The study highlighted the possibility of using the C–C bonds in these vinylidene systems as electron reservoirs, enabling their potential as essential components in nano devices. The compounds were characterized by X-ray diffraction, NMR, IR, and cyclic voltammetry.[15]
References
- ↑ Astruc, Didier; Roman E., Enrique; Hamon, Jean Rene; Batail, Patrick (April 1979). "Novel reactions of dioxygen in organometallic chemistry. Hydrogen atom abstraction vs. dimerization of the 19-electron complexes .eta.5-cyclopentadienyliron(I) .eta.6-arene". Journal of the American Chemical Society. 101 (8): 2240–2242. doi:10.1021/ja00502a070. ISSN 0002-7863.
- 1 2 Astruc, Didier; Hamon, Jean Rene; Althoff, Gisela; Roman, Enrique; Batail, Patrick; Michaud, Pascal; Mariot, Jean Pierre; Varret, Francois; Cozak, Daniel (August 1979). "Design, stabilization, and efficiency of organometallic "electron reservoirs". 19-Electron sandwiches .eta.5-C5R5FeI-.eta.6-C6R'6, a key class active in redox catalysis". Journal of the American Chemical Society. 101 (18): 5445–5447. doi:10.1021/ja00512a071. ISSN 0002-7863.
- ↑ Astruc, Didier (April 2012). "Electron-transfer processes in dendrimers and their implication in biology, catalysis, sensing and nanotechnology". Nature Chemistry. 4 (4): 255–267. Bibcode:2012NatCh...4..255A. doi:10.1038/nchem.1304. ISSN 1755-4349. PMID 22437709.
- ↑ Green, Jennifer C.; Kelly, M. Ruth; Payne, Martin P.; Seddon, Elaine A.; Astruc, Didier; Hamon, Jean Rene; Michaud, Pascal (February 1983). "Photoelectron study of electron-rich iron(I) cyclopentadienyl arene complexes and of the related iron(II) cyclopentadienyl cyclohexadienyl complexes". Organometallics. 2 (2): 211–218. doi:10.1021/om00074a002. ISSN 0276-7333.
- 1 2 3 4 Astruc, Didier (1983-01-01). "Organo-iron complexes of aromatic compounds. Applications in synthesis". Tetrahedron. 39 (24): 4027–4095. doi:10.1016/S0040-4020(01)88627-0. ISSN 0040-4020.
- ↑ Astruc, Didier; Dabard, Rene (26 August 1975). "Elsevier Enhanced Reader". Journal of Organometallic Chemistry. 96 (2): 283–287. doi:10.1016/S0022-328X(00)83560-3. Retrieved 2023-03-17.
- ↑ Hamon, Jean Rene; Astruc, Didier; Michaud, Pascal (February 1981). "Syntheses, characterizations, and stereoelectronic stabilization of organometallic electron reservoirs: the 19-electron d7 redox catalysts .eta.5-C5R5Fe-.eta.6-C6R'6". Journal of the American Chemical Society. 103 (4): 758–766. doi:10.1021/ja00394a006. ISSN 0002-7863.
- ↑ Seyferth, Dietmar (2002-07-01). "Bis(benzene)chromium. 2. Its Discovery by E. O. Fischer and W. Hafner and Subsequent Work by the Research Groups of E. O. Fischer, H. H. Zeiss, F. Hein, C. Elschenbroich, and Others". Organometallics. 21 (14): 2800–2820. doi:10.1021/om020362a. ISSN 0276-7333.
- 1 2 Delville-Desbois, Marie-Hélène; Mross, Stefan; Astruc, Didier; Linares, Jorge; Varret, François; Le Beuze, Albert; Saillard, Jean-Yves; Culp, Robert D.; Atwood, David A.; Cowley, Alan H. (1996-01-01). "17- and 19-Electron Complexes [Fe III (η 5 -C 5 R 5 )(S 2 CNMe 2 )L] n + ( n = 1, 0): Electronic Structure and Substitution and Redox Chemistry. Formation of [Fe IV (η 5 -C 5 R 5 )(dtc) 2 ] and Characterization of both 17e and 19e States of a Transition-Metal Complex". Journal of the American Chemical Society. 118 (17): 4133–4147. doi:10.1021/ja953603x. ISSN 0002-7863.
- ↑ Delville-Desbois, Marie-Hélène; Mross, Stefan; Astruc, Didier; Linares, Jorge; Varret, François; Le Beuze, Albert; Saillard, Jean-Yves; Culp, Robert D.; Atwood, David A.; Cowley, Alan H. (1996-01-01). "17- and 19-Electron Complexes [Fe III (η 5 -C 5 R 5 )(S 2 CNMe 2 )L] n + ( n = 1, 0): Electronic Structure and Substitution and Redox Chemistry. Formation of [Fe IV (η 5 -C 5 R 5 )(dtc) 2 ] and Characterization of both 17e and 19e States of a Transition-Metal Complex". Journal of the American Chemical Society. 118 (17): 4133–4147. doi:10.1021/ja953603x. ISSN 0002-7863.
- ↑ Astruc, Didier; Roman E., Enrique; Hamon, Jean Rene; Batail, Patrick (April 1979). "Novel reactions of dioxygen in organometallic chemistry. Hydrogen atom abstraction vs. dimerization of the 19-electron complexes .eta.5-cyclopentadienyliron(I) .eta.6-arene". Journal of the American Chemical Society. 101 (8): 2240–2242. doi:10.1021/ja00502a070. ISSN 0002-7863.
- 1 2 Méry, Denise; Aranzaes, Jaime Ruiz; Astruc, Didier (2006-05-01). "Use of an Electron-Reservoir Complex Together with Air to Generate N-Heterocyclic Carbenes". Journal of the American Chemical Society. 128 (17): 5602–5603. doi:10.1021/ja058421+. ISSN 0002-7863. PMID 16637604.
- ↑ Arduengo, Anthony J.; Harlow, Richard L.; Kline, Michael (January 1991). "A stable crystalline carbene". Journal of the American Chemical Society. 113 (1): 361–363. doi:10.1021/ja00001a054. ISSN 0002-7863.
- ↑ Chang, Mu-Chieh; Dann, Thomas; Day, David P.; Lutz, Martin; Wildgoose, Gregory G.; Otten, Edwin (2014-04-14). "The Formazanate Ligand as an Electron Reservoir: Bis(Formazanate) Zinc Complexes Isolated in Three Redox States". Angewandte Chemie International Edition. 53 (16): 4118–4122. doi:10.1002/anie.201309948. PMID 24615928.
- ↑ Venkatesan, Koushik; Blacque, Olivier; Fox, Thomas; Alfonso, Montserrat; Schmalle, Helmut W.; Kheradmandan, Sohrab; Berke, Heinz (2005-02-01). "μ-Carbon−Carbon Bonds of Dinuclear Manganese Half-Sandwich Complexes as Electron Reservoirs". Organometallics. 24 (5): 920–932. doi:10.1021/om049284c. ISSN 0276-7333.