 | |
| Names | |
|---|---|
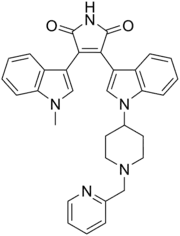
| Preferred IUPAC name
3-(1-Methyl-1H-indol-3-yl)-4-(1-{1-[(pyridin-2-yl)methyl]piperidin-4-yl}-1H-indol-3-yl)-1H-pyrrole-2,5-dione | |
| Other names
LY-317615 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.233.143 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C32H29N5O2 | |
| Molar mass | 515.617 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Enzastaurin is a synthetic bisindolylmaleimide with potential antineoplastic activity. Binding to the ATP-binding site, enzastaurin selectively inhibits protein kinase C beta, an enzyme involved in the induction of vascular endothelial growth factor (VEGF)-stimulated neo-angiogenesis. This agent may decrease tumor blood supply, preventing growth.
Trials
In 2013 it failed a phase III clinical trial for lymphoma.[1]
In 2022, there is an upcoming initial trial called PREVEnt to look into the effectiveness of Enzastaurin for the treatment of Vascular Elhers-Danlos syndrome (vEDS). [2][3][4]
References
- ↑ Lilly Halts Development of Lymphoma Drug After Phase III Failure
- ↑ "New vEDS clinical trial". PREVEnt Trial. Retrieved 2022-01-19.
- ↑ "Aytu BioPharma Adds Late-Stage Pediatric Onset Rare Disease Asset to Development Pipeline from Rumpus Therapeutics". BioSpace. Retrieved 2022-01-19.
- ↑ "Clinical Trials". FIGHT vEDS 3.0. Retrieved 2022-01-19.
External links
- Enzastaurin hydrochloride, National Institutes of Health
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.